Hyperglycemia Does Not Affect Iron Mediated Toxicity of Cultured Endothelial and Renal Tubular Epithelial Cells: Influence of L-Carnosine
- PMID: 26788523
- PMCID: PMC4691606
- DOI: 10.1155/2016/8710432
Hyperglycemia Does Not Affect Iron Mediated Toxicity of Cultured Endothelial and Renal Tubular Epithelial Cells: Influence of L-Carnosine
Abstract
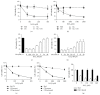
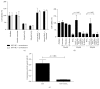
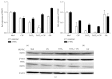
Iron has been suggested to affect the clinical course of type 2 diabetes (T2DM) as accompanying increased intracellular iron accumulation may provide an alternative source for reactive oxygen species (ROS). Although carnosine has proven its therapeutic efficacy in rodent models of T2DM, little is known about its efficacy to protect cells from iron toxicity. We sought to assess if high glucose (HG) exposure makes cultured human umbilical vein endothelial cells (HUVECs) and renal proximal tubular epithelial cells (PTECs) more susceptible to metal induced toxicity and if this is ameliorated by L-carnosine. HUVECs and PTECs, cultured under normal glucose (5 mM, NG) or HG (30 mM), were challenged for 24 h with FeCl3. Cell viability was not impaired under HG conditions nor did HG increase susceptibility to FeCl3. HG did not change the expression of divalent metal transporter 1 (DMT1), ferroportin (IREG), and transferrin receptor protein 1 (TFRC). Irrespective of glucose concentrations L-carnosine prevented toxicity in a dose-dependent manner, only if it was present during the FeCl3 challenge. Hence our study indicates that iron induced cytotoxicity is not enhanced under HG conditions. L-Carnosine displayed a strong protective effect, most likely by chelation of iron mediated toxicity.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

