Reversible Photoinduced Reductive Elimination of H2 from the Nitrogenase Dihydride State, the E(4)(4H) Janus Intermediate
- PMID: 26788586
- PMCID: PMC4773049
- DOI: 10.1021/jacs.5b11650
Reversible Photoinduced Reductive Elimination of H2 from the Nitrogenase Dihydride State, the E(4)(4H) Janus Intermediate
Abstract
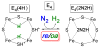
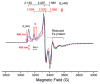
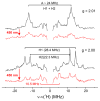
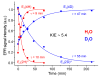
We recently demonstrated that N2 reduction by nitrogenase involves the obligatory release of one H2 per N2 reduced. These studies focus on the E4(4H) "Janus intermediate", which has accumulated four reducing equivalents as two [Fe-H-Fe] bridging hydrides. E4(4H) is poised to bind and reduce N2 through reductive elimination (re) of the two hydrides as H2, coupled to the binding/reduction of N2. To obtain atomic-level details of the re activation process, we carried out in situ 450 nm photolysis of E4(4H) in an EPR cavity at temperatures below 20 K. ENDOR and EPR measurements show that photolysis generates a new FeMo-co state, denoted E4(2H)*, through the photoinduced re of the two bridging hydrides of E4(4H) as H2. During cryoannealing at temperatures above 175 K, E4(2H)* reverts to E4(4H) through the oxidative addition (oa) of the H2. The photolysis quantum yield is temperature invariant at liquid helium temperatures and shows a rather large kinetic isotope effect, KIE = 10. These observations imply that photoinduced release of H2 involves a barrier to the combination of the two nascent H atoms, in contrast to a barrierless process for monometallic inorganic complexes, and further suggest that H2 formation involves nuclear tunneling through that barrier. The oa recombination of E4(2H)* with the liberated H2 offers compelling evidence for the Janus intermediate as the point at which H2 is necessarily lost during N2 reduction; this mechanistically coupled loss must be gated by N2 addition that drives the re/oa equilibrium toward reductive elimination of H2 with N2 binding/reduction.
Figures








Similar articles
-
Photoinduced Reductive Elimination of H2 from the Nitrogenase Dihydride (Janus) State Involves a FeMo-cofactor-H2 Intermediate.Inorg Chem. 2017 Feb 20;56(4):2233-2240. doi: 10.1021/acs.inorgchem.6b02899. Epub 2017 Feb 8. Inorg Chem. 2017. PMID: 28177622 Free PMC article.
-
Reductive Elimination of H2 Activates Nitrogenase to Reduce the N≡N Triple Bond: Characterization of the E4(4H) Janus Intermediate in Wild-Type Enzyme.J Am Chem Soc. 2016 Aug 24;138(33):10674-83. doi: 10.1021/jacs.6b06362. Epub 2016 Aug 16. J Am Chem Soc. 2016. PMID: 27529724 Free PMC article.
-
Time-Resolved EPR Study of H2 Reductive Elimination from the Photoexcited Nitrogenase Janus E4(4H) Intermediate.J Phys Chem B. 2019 Oct 17;123(41):8823-8828. doi: 10.1021/acs.jpcb.9b07776. Epub 2019 Oct 8. J Phys Chem B. 2019. PMID: 31549504
-
Electron Redistribution within the Nitrogenase Active Site FeMo-Cofactor During Reductive Elimination of H2 to Achieve N≡N Triple-Bond Activation.J Am Chem Soc. 2020 Dec 30;142(52):21679-21690. doi: 10.1021/jacs.0c07914. Epub 2020 Dec 16. J Am Chem Soc. 2020. PMID: 33326225 Free PMC article.
-
Breaking the N2 triple bond: insights into the nitrogenase mechanism.Dalton Trans. 2006 May 21;(19):2277-84. doi: 10.1039/b517633f. Epub 2006 Apr 11. Dalton Trans. 2006. PMID: 16688314 Review.
Cited by
-
N2 -to-NH3 Conversion by a triphos-Iron Catalyst and Enhanced Turnover under Photolysis.Angew Chem Int Ed Engl. 2017 Jun 6;56(24):6921-6926. doi: 10.1002/anie.201703244. Epub 2017 May 10. Angew Chem Int Ed Engl. 2017. PMID: 28489303 Free PMC article.
-
Kinetic Understanding of N2 Reduction versus H2 Evolution at the E4(4H) Janus State in the Three Nitrogenases.Biochemistry. 2018 Oct 2;57(39):5706-5714. doi: 10.1021/acs.biochem.8b00784. Epub 2018 Sep 19. Biochemistry. 2018. PMID: 30183278 Free PMC article.
-
Structural Enzymology of Nitrogenase Enzymes.Chem Rev. 2020 Jun 24;120(12):4969-5004. doi: 10.1021/acs.chemrev.0c00067. Epub 2020 Jun 15. Chem Rev. 2020. PMID: 32538623 Free PMC article. Review.
-
Hydride Conformers of the Nitrogenase FeMo-cofactor Two-Electron Reduced State E2(2H), Assigned Using Cryogenic Intra Electron Paramagnetic Resonance Cavity Photolysis.Inorg Chem. 2018 Jun 18;57(12):6847-6852. doi: 10.1021/acs.inorgchem.8b00271. Epub 2018 Mar 24. Inorg Chem. 2018. PMID: 29575898 Free PMC article.
-
Interplay of hemilability and redox activity in models of hydrogenase active sites.Proc Natl Acad Sci U S A. 2017 Nov 14;114(46):E9775-E9782. doi: 10.1073/pnas.1710475114. Epub 2017 Oct 30. Proc Natl Acad Sci U S A. 2017. PMID: 29087322 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

