A Unilateral Cervical Spinal Cord Contusion Injury Model in Non-Human Primates (Macaca mulatta)
- PMID: 26788611
- PMCID: PMC4799702
- DOI: 10.1089/neu.2015.3956
A Unilateral Cervical Spinal Cord Contusion Injury Model in Non-Human Primates (Macaca mulatta)
Abstract
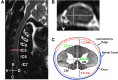
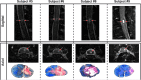
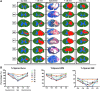
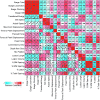
The development of a non-human primate (NHP) model of spinal cord injury (SCI) based on mechanical and computational modeling is described. We scaled up from a rodent model to a larger primate model using a highly controllable, friction-free, electronically-driven actuator to generate unilateral C6-C7 spinal cord injuries. Graded contusion lesions with varying degrees of functional recovery, depending upon pre-set impact parameters, were produced in nine NHPs. Protocols and pre-operative magnetic resonance imaging (MRI) were used to optimize the predictability of outcomes by matching impact protocols to the size of each animal's spinal canal, cord, and cerebrospinal fluid space. Post-operative MRI confirmed lesion placement and provided information on lesion volume and spread for comparison with histological measures. We evaluated the relationships between impact parameters, lesion measures, and behavioral outcomes, and confirmed that these relationships were consistent with our previous studies in the rat. In addition to providing multiple univariate outcome measures, we also developed an integrated outcome metric describing the multivariate cervical SCI syndrome. Impacts at the higher ranges of peak force produced highly lateralized and enduring deficits in multiple measures of forelimb and hand function, while lower energy impacts produced early weakness followed by substantial recovery but enduring deficits in fine digital control (e.g., pincer grasp). This model provides a clinically relevant system in which to evaluate the safety and, potentially, the efficacy of candidate translational therapies.
Keywords: biomechanics of injury; contusion; functional recovery; primate; spinal cord injury.
Figures








Similar articles
-
A controlled spinal cord contusion for the rhesus macaque monkey.Exp Neurol. 2016 May;279:261-273. doi: 10.1016/j.expneurol.2016.02.008. Epub 2016 Feb 11. Exp Neurol. 2016. PMID: 26875994
-
A Cervical Spinal Cord Hemi-Contusion Injury Model Based on Displacement Control in Non-Human Primates (Macaca fascicularis).J Neurotrauma. 2020 Aug 1;37(15):1669-1686. doi: 10.1089/neu.2019.6822. Epub 2020 May 4. J Neurotrauma. 2020. PMID: 32174266
-
Mechanical Design and Analysis of a Unilateral Cervical Spinal Cord Contusion Injury Model in Non-Human Primates.J Neurotrauma. 2016 Jun 15;33(12):1136-49. doi: 10.1089/neu.2015.3974. Epub 2016 Apr 19. J Neurotrauma. 2016. PMID: 26670940 Free PMC article.
-
Animal models of neurologic disorders: a nonhuman primate model of spinal cord injury.Neurotherapeutics. 2012 Apr;9(2):380-92. doi: 10.1007/s13311-012-0114-0. Neurotherapeutics. 2012. PMID: 22427157 Free PMC article. Review.
-
Rodent, large animal and non-human primate models of spinal cord injury.Zoology (Jena). 2017 Aug;123:101-114. doi: 10.1016/j.zool.2017.06.004. Epub 2017 Jun 21. Zoology (Jena). 2017. PMID: 28720322 Review.
Cited by
-
Cell transplantation therapy for spinal cord injury.Nat Neurosci. 2017 Apr 25;20(5):637-647. doi: 10.1038/nn.4541. Nat Neurosci. 2017. PMID: 28440805 Review.
-
Seasonal Prevalence of Gastrointestinal Parasites in Macaques (Macaca thibetana) at Mount Emei Scenic Area in China.Animals (Basel). 2022 Jul 15;12(14):1816. doi: 10.3390/ani12141816. Animals (Basel). 2022. PMID: 35883363 Free PMC article.
-
Targeting Translational Successes through CANSORT-SCI: Using Pet Dogs To Identify Effective Treatments for Spinal Cord Injury.J Neurotrauma. 2017 Jun 15;34(12):2007-2018. doi: 10.1089/neu.2016.4745. Epub 2017 Mar 22. J Neurotrauma. 2017. PMID: 28230415 Free PMC article. Review.
-
Animal Models of Spinal Cord Injury.Biomedicines. 2025 Jun 10;13(6):1427. doi: 10.3390/biomedicines13061427. Biomedicines. 2025. PMID: 40564146 Free PMC article. Review.
-
Correlating Tissue Mechanics and Spinal Cord Injury: Patient-Specific Finite Element Models of Unilateral Cervical Contusion Spinal Cord Injury in Non-Human Primates.J Neurotrauma. 2021 Mar 15;38(6):698-717. doi: 10.1089/neu.2019.6840. Epub 2020 Nov 20. J Neurotrauma. 2021. PMID: 33066716 Free PMC article.
References
-
- Streijger F., Beernink T.M., Lee J.H., Bhatnagar T., Park S., Kwon B.K., and Tetzlaff W. (2013). Characterization of a cervical spinal cord hemicontusion injury in mice using the infinite horizon impactor. J. Neurotrauma 30, 869–883 - PubMed
-
- Russell C.M., Choo A.M., Tetzlaff W., Chung T.E., and Oxland T.R. (2012). Maximum principal strain correlates with spinal cord tissue damage in contusion and dislocation injuries in the rat cervical spine. J. Neurotrauma 29, 1574–1585 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
