Association of Metformin Use with Outcomes in Advanced Endometrial Cancer Treated with Chemotherapy
- PMID: 26788855
- PMCID: PMC4720394
- DOI: 10.1371/journal.pone.0147145
Association of Metformin Use with Outcomes in Advanced Endometrial Cancer Treated with Chemotherapy
Abstract
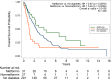
There is increasing evidence that metformin, a commonly used treatment for diabetes, might have the potential to be repurposed as an economical and safe cancer therapeutic. The aim of this study was to determine whether stage III-IV or recurrent endometrial cancer patients who are using metformin during treatment with chemotherapy have improved survival. To test this we analyzed a retrospective cohort of subjects at two independent institutions who received chemotherapy for stage III-IV or recurrent endometrial cancer from 1992 to 2011. Diagnosis of diabetes, metformin use, demographics, endometrial cancer clinico-pathologic parameters, and survival duration were abstracted. The primary outcome was overall survival. The final cohort included 349 patients, 31 (8.9%) had diabetes and used metformin, 28 (8.0%) had diabetes but did not use metformin, and 291 (83.4%) did not have diabetes. The results demonstrate that the median overall survival was 45.6 months for patients with diabetes who used metformin compared to 12.5 months for patients with diabetes who did not use metformin and 28.5 months for patients without diabetes (log-rank test comparing the three groups P = 0.006). In a model adjusted for confounders, the difference in survival between the three groups remained statistically significant (P = 0.023). The improvement in survival among metformin users was not explained by better baseline health status or more aggressive use of chemotherapy. Overall, the findings in this retrospective cohort of endometrial cancer patients with stage III-IV or recurrent disease treated with chemotherapy indicate that patients with diabetes who were concurrently treated with metformin survived longer than patients with diabetes who did not use metformin.
Conflict of interest statement
Figures

References
-
- Sterne J (1957) Du nouveau dans les antidiabetiques. La NN dimethylamine guanyl guanide (N.N.D.G.). Maroc Medical 36: 1295–1296.
-
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. (2012) Management of hyperglycemia in type 2 diabetes: A patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35: 1364–1379. 10.2337/dc12-0413 - DOI - PMC - PubMed
-
- Bowker SL, Majumdar SR, Veugelers P, Johnson JA (2006) Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 29: 254–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000430/TR/NCATS NIH HHS/United States
- NCI T32 CA009566/CA/NCI NIH HHS/United States
- K12 CA139160/CA/NCI NIH HHS/United States
- UL1 RR024999/RR/NCRR NIH HHS/United States
- NCI P50 CA136393/CA/NCI NIH HHS/United States
- 2K12HD000849-26/HD/NICHD NIH HHS/United States
- P50 CA136393/CA/NCI NIH HHS/United States
- NCI CA014599/CA/NCI NIH HHS/United States
- K12 HD000849/HD/NICHD NIH HHS/United States
- P30 CA014599/CA/NCI NIH HHS/United States
- NCI K12 CA139160/CA/NCI NIH HHS/United States
- CTSA-ITM CS UL1 RR024999/RR/NCRR NIH HHS/United States
- T32 CA009566/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

