Chronic Uridine Administration Induces Fatty Liver and Pre-Diabetic Conditions in Mice
- PMID: 26789264
- PMCID: PMC4720477
- DOI: 10.1371/journal.pone.0146994
Chronic Uridine Administration Induces Fatty Liver and Pre-Diabetic Conditions in Mice
Abstract
Uridine is a pyrimidine nucleoside that exerts restorative functions in tissues under stress. Short-term co-administration of uridine with multiple unrelated drugs prevents drug-induced liver lipid accumulation. Uridine has the ability to modulate liver metabolism; however, the precise mechanism has not been delineated. In this study, long-term effects of uridine on liver metabolism were examined in both HepG2 cell cultures and C57BL/6J mice. We report that uridine administration was associated with O-GlcNAc modification of FOXO1, increased gluconeogenesis, reduced insulin signaling activity, and reduced expression of a liver-specific fatty acid binding protein FABP1. Long-term uridine feeding induced systemic glucose intolerance and severe liver lipid accumulation in mice. Our findings suggest that the therapeutic potentials of uridine should be designed for short-term acute administration.
Conflict of interest statement
Figures

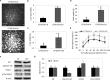
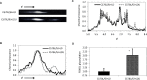
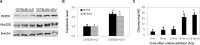
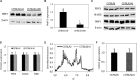
References
-
- Gallai V, Mazzotta G, Montesi S, Sarchielli P, Del Gatto F. Effects of uridine in the treatment of diabetic neuropathy: an electrophysiological study. Acta Neurol Scand 1992;86(1):3–7. - PubMed
-
- Lebrecht D, Vargas-Infante YA, Setzer B, Kirschner J, Walker UA. Uridine supplementation antagonizes zalcitabine-induced microvesicular steatohepatitis in mice. Hepatology 2007;45(1):72–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

