Short Telomeres in Key Tissues Initiate Local and Systemic Aging in Zebrafish
- PMID: 26789415
- PMCID: PMC4720274
- DOI: 10.1371/journal.pgen.1005798
Short Telomeres in Key Tissues Initiate Local and Systemic Aging in Zebrafish
Abstract
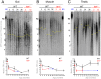
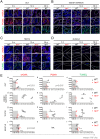
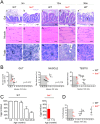
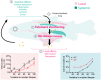
Telomeres shorten with each cell division and telomere dysfunction is a recognized hallmark of aging. Tissue proliferation is expected to dictate the rate at which telomeres shorten. We set out to test whether proliferative tissues age faster than non-proliferative due to telomere shortening during zebrafish aging. We performed a prospective study linking telomere length to tissue pathology and disease. Contrary to expectations, we show that telomeres shorten to critical lengths only in specific tissues and independently of their proliferation rate. Short telomeres accumulate in the gut but not in other highly proliferative tissues such as the blood and gonads. Notably, the muscle, a low proliferative tissue, accumulates short telomeres and DNA damage at the same rate as the gut. Together, our work shows that telomere shortening and DNA damage in key tissues triggers not only local dysfunction but also anticipates the onset of age-associated diseases in other tissues, including cancer.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Olovnikov AM. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. Journal of theoretical biology. 1973;41(1):181–90. . - PubMed
-
- Watson JD. Origin of concatemeric T7 DNA. Nature: New biology. 1972;239(94):197–201. . - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

