CDX2 as a Prognostic Biomarker in Stage II and Stage III Colon Cancer
- PMID: 26789870
- PMCID: PMC4784450
- DOI: 10.1056/NEJMoa1506597
CDX2 as a Prognostic Biomarker in Stage II and Stage III Colon Cancer
Abstract
Background The identification of high-risk stage II colon cancers is key to the selection of patients who require adjuvant treatment after surgery. Microarray-based multigene-expression signatures derived from stem cells and progenitor cells hold promise, but they are difficult to use in clinical practice. Methods We used a new bioinformatics approach to search for biomarkers of colon epithelial differentiation across gene-expression arrays and then ranked candidate genes according to the availability of clinical-grade diagnostic assays. With the use of subgroup analysis involving independent and retrospective cohorts of patients with stage II or stage III colon cancer, the top candidate gene was tested for its association with disease-free survival and a benefit from adjuvant chemotherapy. Results The transcription factor CDX2 ranked first in our screening test. A group of 87 of 2115 tumor samples (4.1%) lacked CDX2 expression. In the discovery data set, which included 466 patients, the rate of 5-year disease-free survival was lower among the 32 patients (6.9%) with CDX2-negative colon cancers than among the 434 (93.1%) with CDX2-positive colon cancers (hazard ratio for disease recurrence, 3.44; 95% confidence interval [CI], 1.60 to 7.38; P=0.002). In the validation data set, which included 314 patients, the rate of 5-year disease-free survival was lower among the 38 patients (12.1%) with CDX2 protein-negative colon cancers than among the 276 (87.9%) with CDX2 protein-positive colon cancers (hazard ratio, 2.42; 95% CI, 1.36 to 4.29; P=0.003). In both these groups, these findings were independent of the patient's age, sex, and tumor stage and grade. Among patients with stage II cancer, the difference in 5-year disease-free survival was significant both in the discovery data set (49% among 15 patients with CDX2-negative tumors vs. 87% among 191 patients with CDX2-positive tumors, P=0.003) and in the validation data set (51% among 15 patients with CDX2-negative tumors vs. 80% among 106 patients with CDX2-positive tumors, P=0.004). In a pooled database of all patient cohorts, the rate of 5-year disease-free survival was higher among 23 patients with stage II CDX2-negative tumors who were treated with adjuvant chemotherapy than among 25 who were not treated with adjuvant chemotherapy (91% vs. 56%, P=0.006). Conclusions Lack of CDX2 expression identified a subgroup of patients with high-risk stage II colon cancer who appeared to benefit from adjuvant chemotherapy. (Funded by the National Comprehensive Cancer Network, the National Institutes of Health, and others.).
Figures

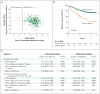
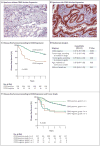


Comment in
-
Data Sharing.N Engl J Med. 2016 Jan 21;374(3):276-7. doi: 10.1056/NEJMe1516564. N Engl J Med. 2016. PMID: 26789876
-
Prognostic Subgroups among Patients with Stage II Colon Cancer.N Engl J Med. 2016 Jan 21;374(3):277-8. doi: 10.1056/NEJMe1514353. N Engl J Med. 2016. PMID: 26789877 Free PMC article.
-
Prediction of chemotherapy benefit for colon cancers.Lancet Oncol. 2016 Mar;17(3):e91. doi: 10.1016/S1470-2045(16)00068-1. Epub 2016 Jan 29. Lancet Oncol. 2016. PMID: 26831289 No abstract available.
-
CDX2: Linking Cell and Patient Fates in Colon Cancer.Cell Stem Cell. 2016 Feb 4;18(2):168-9. doi: 10.1016/j.stem.2016.01.011. Cell Stem Cell. 2016. PMID: 26849302 Free PMC article.
-
Gastrointestinal cancer: CDX2: prognostic marker for high-risk colon cancer.Nat Rev Clin Oncol. 2016 Mar;13(3):134-5. doi: 10.1038/nrclinonc.2016.18. Epub 2016 Feb 16. Nat Rev Clin Oncol. 2016. PMID: 26880474 No abstract available.
-
CDX2 as a Prognostic Biomarker in Colon Cancer.N Engl J Med. 2016 Jun 2;374(22):2184. doi: 10.1056/NEJMc1602584. N Engl J Med. 2016. PMID: 27248627 Free PMC article. No abstract available.
-
CDX2 as a Prognostic Biomarker in Colon Cancer.N Engl J Med. 2016 Jun 2;374(22):2182. doi: 10.1056/NEJMc1602584. N Engl J Med. 2016. PMID: 27248628 No abstract available.
-
CDX2 as a Prognostic Biomarker in Colon Cancer.N Engl J Med. 2016 Jun 2;374(22):2183. doi: 10.1056/NEJMc1602584. N Engl J Med. 2016. PMID: 27248629 Free PMC article. No abstract available.
-
CDX2 as a Prognostic Biomarker in Colon Cancer.N Engl J Med. 2016 Jun 2;374(22):2183-4. doi: 10.1056/NEJMc1602584. N Engl J Med. 2016. PMID: 27248630 No abstract available.
-
Curing more colorectal cancer.Natl Med J India. 2016 May-Jun;29(3):155-157. Natl Med J India. 2016. PMID: 27808066 No abstract available.
-
Correction to our Letter to the Editor about "CDX2 as a Prognostic Biomarker in Stage II and Stage III Colon Cancer".N Engl J Med. 2018 Dec 20;379(25):2481. doi: 10.1056/NEJMc1814750. N Engl J Med. 2018. PMID: 30575475 No abstract available.
References
-
- André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–51. - PubMed
-
- Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352:476–87. - PubMed
-
- Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2000;343:905–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 CA180868/CA/NCI NIH HHS/United States
- U24-CA114732/CA/NCI NIH HHS/United States
- UG1 CA189867/CA/NCI NIH HHS/United States
- P01-CA139490/CA/NCI NIH HHS/United States
- R00-CA151673/CA/NCI NIH HHS/United States
- U10-CA180868/CA/NCI NIH HHS/United States
- U24 CA114732/CA/NCI NIH HHS/United States
- U54 CA126524/CA/NCI NIH HHS/United States
- R00 CA151673/CA/NCI NIH HHS/United States
- U24 CA196067/CA/NCI NIH HHS/United States
- U54-CA126524/CA/NCI NIH HHS/United States
- U10-CA180822/CA/NCI NIH HHS/United States
- U10-CA37377/CA/NCI NIH HHS/United States
- P01 CA139490/CA/NCI NIH HHS/United States
- UG1-CA18986/CA/NCI NIH HHS/United States
- U10 CA180822/CA/NCI NIH HHS/United States
- U10 CA037377/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
