Lack of MG53 in human heart precludes utility as a biomarker of myocardial injury or endogenous cardioprotective factor
- PMID: 26790476
- PMCID: PMC4836626
- DOI: 10.1093/cvr/cvw017
Lack of MG53 in human heart precludes utility as a biomarker of myocardial injury or endogenous cardioprotective factor
Abstract
Aims: Mitsugumin-53 (MG53/TRIM72) is an E3-ubiquitin ligase that rapidly accumulates at sites of membrane injury and plays an important role in membrane repair of skeletal and cardiac muscle. MG53 has been implicated in cardiac ischaemia-reperfusion injury, and serum MG53 provides a biomarker of skeletal muscle injury in the mdx mouse model of Duchenne muscular dystrophy. We evaluated the clinical utility of MG53 as a biomarker of myocardial injury.
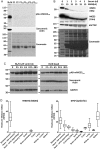
Methods and results: We performed Langendorff ischaemia-reperfusion injury on wild-type and dysferlin-null murine hearts, using dysferlin deficiency to effectively model more severe outcomes from cardiac ischaemia-reperfusion injury. MG53 released into the coronary effluent correlated strongly and significantly (r = 0.79-0.85, P < 0.0001) with functional impairment after ischaemic injury. We initiated a clinical trial in paediatric patients undergoing corrective heart surgery, the first study of MG53 release with myocardial injury in humans. Unexpectedly, we reveal although MG53 is robustly expressed in rat and mouse hearts, MG53 is scant to absent in human, ovine, or porcine hearts. Absence of MG53 in 11 human heart specimens was confirmed using three separate antibodies to MG53, each subject to epitope mapping and confirmed immunospecificity using MG53-deficient muscle cells.
Conclusion: MG53 is an effective biomarker of myocardial injury and dysfunction in murine hearts. However, MG53 is not expressed in human heart and therefore does not hold utility as a clinical biomarker of myocardial injury. Although cardioprotective roles for endogenous myocardial MG53 cannot be extrapolated from rodents to humans, potential therapeutic application of recombinant MG53 for myocardial membrane injury prevails.
Keywords: Biomarker of myocardial injury; Ischaemia–reperfusion injury; Ischaemic preconditioning/postconditioning; MG53; TRIM72.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2016. For permissions please email: journals.permissions@oup.com.
Figures





References
-
- Waddell LB, Lemckert FA, Zheng XF, Tran J, Evesson FJ, Hawkes JM, Lek A, Street NE, Lin P, Clarke NF, Landstrom AP, Ackerman MJ, Weisleder N, Ma J, North KN, Cooper ST. Dysferlin, annexin A1, and mitsugumin 53 are upregulated in muscular dystrophy and localize to longitudinal tubules of the T-system with stretch. J Neuropath Exp Neur 2011;70:302–313. - PMC - PubMed
-
- Wang X, Xie W, Zhang Y, Lin P, Han L, Han P, Wang Y, Chen Z, Ji G, Zheng M, Weisleder N, Xiao R-P, Takeshima H, Ma J, Cheng H. Cardioprotection of ischemia/reperfusion injury by cholesterol-dependent MG53-mediated membrane repair. Circ Res 2010;107:76–83. - PubMed
-
- Cao C-M, Zhang Y, Weisleder N, Ferrante C, Wang X, Lv F, Zhang Y, Song R, Hwang M, Jin L, Guo J, Peng W, Li G, Nishi M, Takeshima H, Ma J, Xiao R-P. MG53 constitutes a primary determinant of cardiac ischemic preconditioning. Circulation 2010;121:2565–2574. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

