Neutralizing Antibodies Against Adeno-Associated Viral Capsids in Patients with mut Methylmalonic Acidemia
- PMID: 26790480
- PMCID: PMC4841085
- DOI: 10.1089/hum.2015.092
Neutralizing Antibodies Against Adeno-Associated Viral Capsids in Patients with mut Methylmalonic Acidemia
Abstract
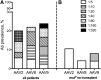
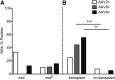
Isolated methylmalonic acidemia (MMA), a group of autosomal recessive inborn errors of metabolism, is most commonly caused by complete (mut(0)) or partial (mut(-)) deficiency of the enzyme methylmalonyl-CoA mutase (MUT). The severe metabolic instability and increased mortality experienced by many affected individuals, especially those with mut(0) MMA, has led centers to use elective liver transplantation as a treatment for these patients. We have previously demonstrated the efficacy of systemic adeno-associated viral (AAV) gene delivery as a treatment for MMA in a murine model and therefore sought to survey AAV antibody titers against serotypes 2, 8, and 9 in a group of well-characterized MMA patients, accrued via a dedicated natural history study ( clinicaltrials.gov ID: NCT00078078). Plasma samples provided by 42 patients (8 mut(-) and 34 mut(0); 10 had received organ transplantation), who ranged in age between 2 and 31 years, were analyzed to examine AAV2 (n = 35), AAV8 (n = 41), and AAV9 (n = 42) antibody titers. In total, the seroprevalence of antibodies against AAV2, AAV8, or AAV9 was 20%, 22%, and 24%, respectively. We observed a lower-than-expected seropositivity rate (titers ≥1:20) in the pediatric MMA patients (2-18 years) for both AAV2 (p < 0.05) and AAV8 (p < 0.01) neutralizing antibodies (NAbs) compared with historical controls. Those with positive NAb titers were typically older than 18 years (p < 0.05 all serotypes) or had received solid organ transplantation (p < 0.01 AAV8, AAV9). The mut(0) patients who had not been transplanted (n = 24)-that is, the subset with the greatest need for improved treatments-represented the seronegative majority, with 21 out of 24 patients lacking Abs against all AAV capsids tested. The unexpected lack of NAbs against AAV in this patient population has encouraging implications for systemic gene delivery as a treatment for mut MMA.
Figures

References
-
- Fenton WA, Gravel RA, Rosenblatt DS. Disorders of propionate and methylmalonate metabolism in the metabolic and molecular bases for inherited disease. In: The Metabolic and Molecular Bases of Inherited Disease (8th edn.). Scriver CR, Beaudet AL, Sly WS, Valle D, eds. McGraw-Hill, New York, NY: 2001; pp. 2165–2192
-
- Manoli I, Sloan JL, Venditti CP. Isolated Methylmalonic Acidemia. In: GeneReviews® [Internet]. Pagon RA, Adam MP, Ardinger HH, et al., eds. University of Washington, Seattle, WA: 2005. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1231/ - PubMed
-
- Matsui SM, Mahoney MJ, Rosenberg LE. The natural history of the inherited methylmalonic acidemias. N Engl J Med 1983;308:857–861 - PubMed
-
- van der Meer SB, Poggi F, Spada M, et al. . Clinical outcome of long-term management of patients with vitamin B12-unresponsive methylmalonic acidemia. J Pediatr 1994;125:903–908 - PubMed
-
- Horster F, Baumgartner MR, Viardot C, et al. . Long-term outcome in methylmalonic acidurias is influenced by the underlying defect (mut0, mut-, cblA, cblB). Pediatr Res 2007;62:225–230 - PubMed
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

