Rab11-FIP1A regulates early trafficking into the recycling endosomes
- PMID: 26790954
- PMCID: PMC4744548
- DOI: 10.1016/j.yexcr.2016.01.003
Rab11-FIP1A regulates early trafficking into the recycling endosomes
Abstract
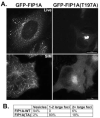
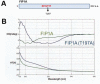
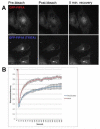
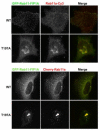
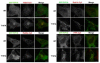
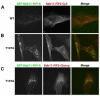
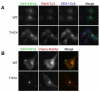
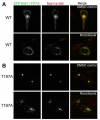
The Rab11 family of small GTPases, along with the Rab11-family interacting proteins (Rab11-FIPs), are critical regulators of intracellular vesicle trafficking and recycling. We have identified a point mutation of Threonine-197 site to an Alanine in Rab11-FIP1A, which causes a dramatic dominant negative phenotype when expressed in HeLa cells. The normally perinuclear distribution of GFP-Rab11-FIP1A was condensed into a membranous cisternum with almost no GFP-Rab11-FIP1A(T197A) remaining outside of this central locus. Also, this condensed GFP-FIP1A(T197A) altered the distribution of proteins in the Rab11a recycling pathway including endogenous Rab11a, Rab11-FIP1C, and transferrin receptor (CD71). Furthermore, this condensed GFP-FIP1A(T197A)-containing structure exhibited little movement in live HeLa cells. Expression of GFP-FIP1A(T197A) caused a strong blockade of transferrin recycling. Treatment of cells expressing GFP-FIP1A(T197A) with nocodazole did not disperse the Rab11a-containing recycling system. We also found that Rab5 and EEA1 were accumulated in membranes by GFP-Rab11-FIP1A but Rab4 was unaffected, suggesting that a direct pathway may exist from early endosomes into the Rab11a-containing recycling system. Our study of a potent inhibitory trafficking mutation in Rab11-FIP1A shows that Rab11-FIP1A associates with and regulates trafficking at an early step in the process of membrane recycling.
Keywords: EEA1; Endocytosis; Membrane recycling; Rab11; Rab11-FIP1; Rab11-FIP2; Rab11-FIP5; Rab11a; Rab14; Rab4; Rab5; Rab8a.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



References
-
- Schwartz SL, Cao C, Pylypenko O, Rak A, Wandinger-Ness A. Rab GTPases at a glance. J Cell Sci. 2007;120:3905–3910. - PubMed
-
- Green EG, Ramm E, Riley NM, Spiro DJ, Goldenring JR, Wessling-Resnick M. Rab11 is associated with transferrin-containing recycling compartments in K562 cells. Biochem.Biophys.Res.Commun. 1997;239:612–616. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

