Presynaptic, release-regulating mGlu2 -preferring and mGlu3 -preferring autoreceptors in CNS: pharmacological profiles and functional roles in demyelinating disease
- PMID: 26791341
- PMCID: PMC4831303
- DOI: 10.1111/bph.13442
Presynaptic, release-regulating mGlu2 -preferring and mGlu3 -preferring autoreceptors in CNS: pharmacological profiles and functional roles in demyelinating disease
Abstract
Background and purpose: Presynaptic, release-regulating metabotropic glutamate 2 and 3 (mGlu2/3) autoreceptors exist in the CNS. They represent suitable targets for therapeutic approaches to central diseases that are typified by hyperglutamatergicity. The availability of specific ligands able to differentiate between mGlu2 and mGlu3 subunits allows us to further characterize these autoreceptors. In this study we investigated the pharmacological profile of mGlu2/3 receptors in selected CNS regions and evaluated their functions in mice with experimental autoimmune encephalomyelitis (EAE).
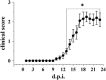
Experimental approach: The comparative analysis of presynaptic mGlu2/3 autoreceptors was performed by determining the effect of selective mGlu2/3 receptor agonist(s) and antagonist(s) on the release of [(3)H]-D-aspartate from cortical and spinal cord synaptosomes in superfusion. In EAE mice, mGlu2/3 autoreceptor-mediated release functions were investigated and effects of in vivo LY379268 administration on impaired glutamate release examined ex vivo.
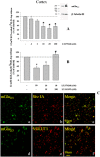
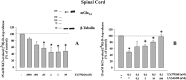
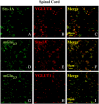
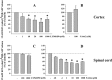
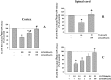
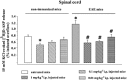
Key results: Western blot analysis and confocal microscopy confirmed the presence of presynaptic mGlu2/3 receptor proteins. Cortical synaptosomes possessed LY541850-sensitive, NAAG-insensitive autoreceptors having low affinity for LY379268, while LY541850-insensitive, NAAG-sensitive autoreceptors with high affinity for LY379268 existed in spinal cord terminals. In EAE mice, mGlu2/3 autoreceptors completely lost their inhibitory activity in cortical, but not in spinal cord synaptosomes. In vivo LY379268 administration restored the glutamate exocytosis capability in spinal cord but not in cortical terminals in EAE mice.
Conclusions and implications: We propose the existence of mGlu2-preferring and mGlu3-preferring autoreceptors in mouse cortex and spinal cord respectively. The mGlu3 -preferring autoreceptors could represent a target for new pharmacological approaches for treating demyelinating diseases.
© 2016 The British Pharmacological Society.
Figures
Similar articles
-
Immuno-pharmacological characterization of group II metabotropic glutamate receptors controlling glutamate exocytosis in mouse cortex and spinal cord.Br J Pharmacol. 2017 Dec;174(24):4785-4796. doi: 10.1111/bph.14061. Epub 2017 Oct 29. Br J Pharmacol. 2017. PMID: 28967122 Free PMC article.
-
5-HT2A-mGlu2/3 receptor complex in rat spinal cord glutamatergic nerve endings: A 5-HT2A to mGlu2/3 signalling to amplify presynaptic mechanism of auto-control of glutamate exocytosis.Neuropharmacology. 2018 May 1;133:429-439. doi: 10.1016/j.neuropharm.2018.02.030. Epub 2018 Feb 27. Neuropharmacology. 2018. PMID: 29499271
-
Differentiating the roles of mGlu2 and mGlu3 receptors using LY541850, an mGlu2 agonist/mGlu3 antagonist.Neuropharmacology. 2013 Mar;66:114-21. doi: 10.1016/j.neuropharm.2012.02.023. Epub 2012 Mar 15. Neuropharmacology. 2013. PMID: 22445601
-
Pharmacological and molecular characterization of the positive allosteric modulators of metabotropic glutamate receptor 2.Neuropharmacology. 2016 Dec;111:253-265. doi: 10.1016/j.neuropharm.2016.08.032. Epub 2016 Aug 30. Neuropharmacology. 2016. PMID: 27590915 Review.
-
Progress in the developement of positive allosteric modulators of the metabotropic glutamate receptor 2.Curr Med Chem. 2011;18(1):47-68. doi: 10.2174/092986711793979706. Curr Med Chem. 2011. PMID: 21110815 Review.
Cited by
-
Immuno-pharmacological characterization of group II metabotropic glutamate receptors controlling glutamate exocytosis in mouse cortex and spinal cord.Br J Pharmacol. 2017 Dec;174(24):4785-4796. doi: 10.1111/bph.14061. Epub 2017 Oct 29. Br J Pharmacol. 2017. PMID: 28967122 Free PMC article.
-
Prophylactic versus Therapeutic Fingolimod: Restoration of Presynaptic Defects in Mice Suffering from Experimental Autoimmune Encephalomyelitis.PLoS One. 2017 Jan 26;12(1):e0170825. doi: 10.1371/journal.pone.0170825. eCollection 2017. PLoS One. 2017. PMID: 28125677 Free PMC article.
-
Scaffold Hopping to Imidazo[1,2-a]pyrazin-8-one Positive Allosteric Modulators of Metabotropic Glutamate 2 Receptor.ACS Omega. 2021 Aug 25;6(35):22997-23006. doi: 10.1021/acsomega.1c03739. eCollection 2021 Sep 7. ACS Omega. 2021. PMID: 34514269 Free PMC article.
-
Presynaptic Release-Regulating Alpha2 Autoreceptors: Potential Molecular Target for Ellagic Acid Nutraceutical Properties.Antioxidants (Basel). 2021 Nov 4;10(11):1759. doi: 10.3390/antiox10111759. Antioxidants (Basel). 2021. PMID: 34829630 Free PMC article.
-
CCL5-Glutamate Cross-Talk in Astrocyte-Neuron Communication in Multiple Sclerosis.Front Immunol. 2017 Sep 4;8:1079. doi: 10.3389/fimmu.2017.01079. eCollection 2017. Front Immunol. 2017. PMID: 28928746 Free PMC article. Review.
References
-
- Bockaert J, Fagni L, Pin J‐P (2002). Metabotropic glutamate receptors (mGluRs). Structure and function In: Egebjerg J, Schousboe A, Krogsgaard‐Larsen P. (eds). Glutamate and GABA Receptors and Transporters. Structure, Function and Pharmacology. Taylor & Francis: London and New York, pp. 121–150.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

