A study of an intelligent system to support decision making in the management of labour using the cardiotocograph - the INFANT study protocol
- PMID: 26791569
- PMCID: PMC4719576
- DOI: 10.1186/s12884-015-0780-0
A study of an intelligent system to support decision making in the management of labour using the cardiotocograph - the INFANT study protocol
Abstract
Background: Continuous electronic fetal heart rate monitoring in labour is widely used but its potential for improving fetal and neonatal outcomes has not been realised. The most likely reason is the difficulty of interpreting the fetal heart rate trace correctly during labour. Computerised interpretation of the fetal heart rate and intelligent decision-support has the potential to deliver this improvement in care. This trial will test whether the addition of decision support software to aid the interpretation of the cardiotocogram (CTG) during labour will reduce the number of 'poor neonatal outcomes' in those women judged to require continuous electronic fetal heart rate monitoring.
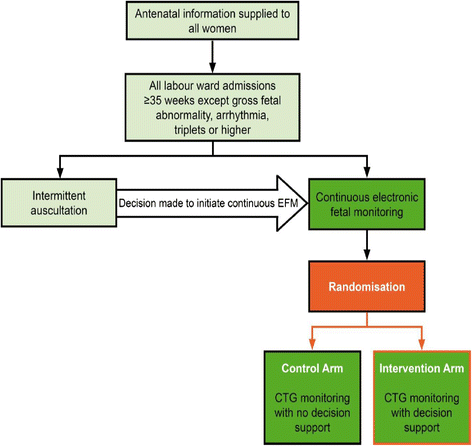
Methods and design: An individually randomised controlled trial of 46,000 women who are judged to require continuous electronic fetal monitoring in labour.
Eligibility criteria: Women admitted to a participating labour ward who are judged to require continuous electronic fetal monitoring, have a singleton or twin pregnancy, are ≥ 35 weeks' gestation, have no known gross fetal abnormality and are ≥ 16 years of age.
Exclusion criteria: Triplets or higher order pregnancy, elective caesarean section prior to the onset of labour, planned admission to NICU. Trial interventions: Computerised interpretation of the CTG with decision-support.
Primary outcomes: Short term: A composite of 'poor neonatal outcome' including stillbirth after trial entry, early neonatal death except deaths due to congenital anomalies, significant morbidity: neonatal encephalopathy, admissions to the neonatal unit with 48 h for > 48 h with evidence of feeding difficulties, respiratory illness or encephalopathy where there is evidence of compromise at birth. Long term: Developmental assessment at the age of 2 years in a subset of 7000 surviving babies.
Data collection: For all participating women and babies, labour variables and outcomes will be stored automatically and contemporaneously onto the Guardian® system.
Discussion: The results of this trial will have importance for pregnant women and for health professionals who provide care for them.
Trial registration: Current Controlled Trials ISRCTN98680152 assigned 30.09.2008.
Figures
References
-
- Levene MI. The asphyxiated newborn infant. In: Levene MI, Lilford RJ, Bennett MJ, Punt J, editors. Fetal and Neonatal Neurology and Neurosurgery. London: Churchill Livingstone; 1995. pp. 405–425.
-
- Volpe JJ. Neurology of the Newborn. In: Volpe JJ, editor. Neurology of the Newborn. Philadelphia: WB Saunders; 1994. pp. 314–369.
-
- Macfarlane A, Johnson A, Mugford M. Epidemiology. In: Rennie JM, Roberton NRC, editors. Textbook of Neonatology. 3. London: Churchill Livingstone; 1999. pp. 3–33.
-
- Hon EH. The classification of fetal heart rate. I. A working classification. Obstet Gynecol. 1963;22:137–46. - PubMed
-
- Hon EH. Instrumentation of fetal heart rate and fetal electrocardiography II. A vaginal electrode. Am J Obstet Gynecol. 1963;86:772–84. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical