Reparative inflammation takes charge of tissue regeneration
- PMID: 26791721
- PMCID: PMC5228603
- DOI: 10.1038/nature17039
Reparative inflammation takes charge of tissue regeneration
Abstract
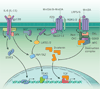
Inflammation underlies many chronic and degenerative diseases, but it also mitigates infections, clears damaged cells and initiates tissue repair. Many of the mechanisms that link inflammation to damage repair and regeneration in mammals are conserved in lower organisms, indicating that it is an evolutionarily important process. Recent insights have shed light on the cellular and molecular processes through which conventional inflammatory cytokines and Wnt factors control mammalian tissue repair and regeneration. This is particularly important for regeneration in the gastrointestinal system, especially for intestine and liver tissues in which aberrant and deregulated repair results in severe pathologies.
Figures

References
-
- Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–284. - PubMed
-
-
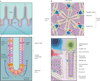
Sato T, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. This paper outlines how Paneth cells provide support for ISCs.
-
-
-
Wang B, Zhao L, Fish M, Logan CY, Nusse R. Self-renewing diploid Axin2+ cells fuel homeostatic renewal of the liver. Nature. 2015;524:180–185. This paper describes a population of diploid pericentral hepatocytes that may act as adult liver stem cells.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

