Angiocrine functions of organ-specific endothelial cells
- PMID: 26791722
- PMCID: PMC4878406
- DOI: 10.1038/nature17040
Angiocrine functions of organ-specific endothelial cells
Abstract
Endothelial cells that line capillaries are not just passive conduits for delivering blood. Tissue-specific endothelium establishes specialized vascular niches that deploy sets of growth factors, known as angiocrine factors. These cues participate actively in the induction, specification, patterning and guidance of organ regeneration, as well as in the maintainance of homeostasis and metabolism. When upregulated following injury, they orchestrate self-renewal and differentiation of tissue-specific resident stem and progenitor cells into functional organs. Uncovering the mechanisms by which organotypic endothelium distributes physiological levels of angiocrine factors both spatially and temporally will lay the foundation for clinical trials that promote organ repair without scarring.
Figures
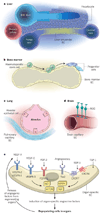


References
-
- Ghesquiere B, Wong BW, Kuchnio A, Carmeliet P. Metabolism of stromal and immune cells in health and disease. Nature. 2014;511:167–176. - PubMed
-
-
Nolan DJ, et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell. 2013;26:204–219. In this article the authors developed an intra-vital labeling approach to purify non-lymphatic mouse organ-specific ECs and by employing molecular profiling demonstrated the remarkable angiocrine heterogeneity in ECs among various tissues
-
-
- Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

