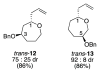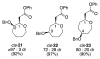Stereoelectronic Model To Explain Highly Stereoselective Reactions of Seven-Membered-Ring Oxocarbenium-Ion Intermediates
- PMID: 26791884
- PMCID: PMC4811184
- DOI: 10.1002/anie.201507806
Stereoelectronic Model To Explain Highly Stereoselective Reactions of Seven-Membered-Ring Oxocarbenium-Ion Intermediates
Abstract
Nucleophilic attack on seven-membered-ring oxocarbenium ions is generally highly stereoselective. The preferred mode of nucleophilic attack forms the product in a conformation that minimizes transannular interactions, thus leading to different stereoselectivity as compared to that of reactions involving six-membered-ring oxocarbenium ions.
Keywords: carbocations; carbohydrates; conformational analysis; electrostatic effects; stereoselectivity.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Similar articles
-
The Experimental Evidence in Support of Glycosylation Mechanisms at the SN1-SN2 Interface.Chem Rev. 2018 Sep 12;118(17):8242-8284. doi: 10.1021/acs.chemrev.8b00083. Epub 2018 May 30. Chem Rev. 2018. PMID: 29846062 Free PMC article. Review.
-
Using stereoelectronic effects to explain selective reactions of 4-substituted five-membered ring oxocarbenium ions.Org Lett. 2004 Jun 10;6(12):2063-6. doi: 10.1021/ol0492647. Org Lett. 2004. PMID: 15176819
-
Using nucleophilic substitution reactions to understand how a remote alkyl or alkoxy substituent influences the conformation of eight-membered ring oxocarbenium ions.Org Lett. 2004 Dec 9;6(25):4739-41. doi: 10.1021/ol047998d. Org Lett. 2004. PMID: 15575674
-
Nucleophilic additions of trimethylsilyl cyanide to cyclic oxocarbenium ions: evidence for the loss of stereoselectivity at the limits of diffusion control.J Am Chem Soc. 2006 Jul 5;128(26):8671-7. doi: 10.1021/ja061110u. J Am Chem Soc. 2006. PMID: 16802834
-
Glycosyl Oxocarbenium Ions: Structure, Conformation, Reactivity, and Interactions.Acc Chem Res. 2021 Jun 1;54(11):2552-2564. doi: 10.1021/acs.accounts.1c00021. Epub 2021 Apr 30. Acc Chem Res. 2021. PMID: 33930267 Free PMC article. Review.
Cited by
-
Oxo-Rhenium-Mediated Allylation of Furanoside Derivatives: A Computational Study on the Mechanism and the Stereoselectivity.J Org Chem. 2022 Aug 5;87(15):9497-9506. doi: 10.1021/acs.joc.2c00393. Epub 2022 Jul 12. J Org Chem. 2022. PMID: 35820228 Free PMC article.
-
CF3-substituted carbocations: underexploited intermediates with great potential in modern synthetic chemistry.Beilstein J Org Chem. 2021 Feb 3;17:343-378. doi: 10.3762/bjoc.17.32. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 33828616 Free PMC article. Review.
-
Highly Stereoselective Glycosylation Reactions of Furanoside Derivatives via Rhenium (V) Catalysis.J Org Chem. 2021 Jun 4;86(11):7672-7686. doi: 10.1021/acs.joc.1c00706. Epub 2021 May 25. J Org Chem. 2021. PMID: 34033490 Free PMC article.
-
Acetal Substitution Reactions: Stereoelectronic Effects, Conformational Analysis, Reactivity vs. Selectivity, and Neighboring-Group Participation.Synlett. 2024 Sep;35(15):1763-1787. doi: 10.1055/s-0042-1751541. Epub 2024 Jan 16. Synlett. 2024. PMID: 39502501 Free PMC article.
-
The Experimental Evidence in Support of Glycosylation Mechanisms at the SN1-SN2 Interface.Chem Rev. 2018 Sep 12;118(17):8242-8284. doi: 10.1021/acs.chemrev.8b00083. Epub 2018 May 30. Chem Rev. 2018. PMID: 29846062 Free PMC article. Review.
References
-
- Kleinke AS, Webb D, Jamison TF. Tetrahedron. 2012;68:6999–7018.
-
- Saha J, Peczuh MW. In: Advances in Carbohydrate Chemistry and Biochemistry. Derek H, editor. Vol. 66. Academic; 2011. pp. 121–186. - PubMed
-
-
See, for example: Nicolaou KC, Hwang CK, Duggan ME, Nugiel DA, Abe Y, Reddy KB, DeFrees SA, Reddy DR, Awartani RA, Conley SR, Rutjes FPJT, Theodorakis EA. J Am Chem Soc. 1995;117:10227–10238.Castro S, Fyvie WS, Hatcher SA, Peczuh MW. Org Lett. 2005;7:4709–4712.Boone MA, McDonald FE, Lichter J, Lutz S, Cao R, Hardcastle KI. Org Lett. 2009;11:851–854.Fuwa H, Ishigai K, Hashizume K, Sasaki M. J Am Chem Soc. 2012;134:11984–11987.
-
-
- Entrena A, Campos JM, Gallo MA, Espinosa A. ARKIVOC. 2005;vi:88–108.
-
- Espinosa A, Gallo MA, Entrena A, Gómez JA. J Mol Struct. 1994;323:247–256.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources