Production, Purification, and Characterization of ¹⁵N-Labeled DNA Repair Proteins as Internal Standards for Mass Spectrometric Measurements
- PMID: 26791985
- PMCID: PMC4888786
- DOI: 10.1016/bs.mie.2015.06.044
Production, Purification, and Characterization of ¹⁵N-Labeled DNA Repair Proteins as Internal Standards for Mass Spectrometric Measurements
Abstract
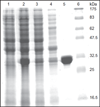
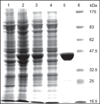
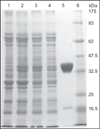
Oxidatively induced DNA damage is caused in living organisms by a variety of damaging agents, resulting in the formation of a multiplicity of lesions, which are mutagenic and cytotoxic. Unless repaired by DNA repair mechanisms before DNA replication, DNA lesions can lead to genomic instability, which is one of the hallmarks of cancer. Oxidatively induced DNA damage is mainly repaired by base excision repair pathway with the involvement of a plethora of proteins. Cancer tissues develop greater DNA repair capacity than normal tissues by overexpressing DNA repair proteins. Increased DNA repair in tumors that removes DNA lesions generated by therapeutic agents before they became toxic is a major mechanism in the development of therapy resistance. Evidence suggests that DNA repair capacity may be a predictive biomarker of patient response. Thus, knowledge of DNA-protein expressions in disease-free and cancerous tissues may help predict and guide development of treatments and yield the best therapeutic response. Our laboratory has developed methodologies that use mass spectrometry with isotope dilution for the measurement of expression of DNA repair proteins in human tissues and cultured cells. For this purpose, full-length (15)N-labeled analogs of a number of human DNA repair proteins have been produced and purified to be used as internal standards for positive identification and accurate quantification. This chapter describes in detail the protocols of this work. The use of (15)N-labeled proteins as internal standards for the measurement of several DNA repair proteins in vivo is also presented.
Keywords: (15)N-labeled proteins; DNA damage; DNA repair; DNA repair proteins; Mass spectrometry; Protein measurements.
© 2016 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Identification and quantification of DNA repair proteins by liquid chromatography/isotope-dilution tandem mass spectrometry using their fully 15N-labeled analogues as internal standards.J Proteome Res. 2011 Aug 5;10(8):3802-13. doi: 10.1021/pr200269j. Epub 2011 Jun 20. J Proteome Res. 2011. PMID: 21619077
-
Oxidatively induced DNA damage and its repair in cancer.Mutat Res Rev Mutat Res. 2015 Jan-Mar;763:212-45. doi: 10.1016/j.mrrev.2014.11.002. Epub 2014 Nov 25. Mutat Res Rev Mutat Res. 2015. PMID: 25795122 Review.
-
Stable isotope-labeling of DNA repair proteins, and their purification and characterization.Protein Expr Purif. 2011 Jul;78(1):94-101. doi: 10.1016/j.pep.2011.02.011. Epub 2011 Feb 26. Protein Expr Purif. 2011. PMID: 21356311
-
Identification and quantification of human DNA repair protein NEIL1 by liquid chromatography/isotope-dilution tandem mass spectrometry.J Proteome Res. 2013 Feb 1;12(2):1049-61. doi: 10.1021/pr301037t. Epub 2013 Jan 11. J Proteome Res. 2013. PMID: 23268652
-
Measurement of oxidatively induced DNA damage and its repair, by mass spectrometric techniques.Free Radic Res. 2015 May;49(5):525-48. doi: 10.3109/10715762.2015.1014814. Epub 2015 Mar 27. Free Radic Res. 2015. PMID: 25812590 Review.
Cited by
-
Biomarker Assay Validation by Mass Spectrometry.AAPS J. 2022 May 9;24(3):66. doi: 10.1208/s12248-022-00707-z. AAPS J. 2022. PMID: 35534647
-
Measuring biological aging in humans: A quest.Aging Cell. 2020 Feb;19(2):e13080. doi: 10.1111/acel.13080. Epub 2019 Dec 12. Aging Cell. 2020. PMID: 31833194 Free PMC article. Review.
-
Inhibition of human APE1 and MTH1 DNA repair proteins by dextran-coated γ-Fe2O3 ultrasmall superparamagnetic iron oxide nanoparticles.Nanomedicine (Lond). 2022 Nov;17(26):2011-2021. doi: 10.2217/nnm-2022-0204. Epub 2023 Feb 28. Nanomedicine (Lond). 2022. PMID: 36853189 Free PMC article.
-
Repair of oxidatively induced DNA damage by DNA glycosylases: Mechanisms of action, substrate specificities and excision kinetics.Mutat Res Rev Mutat Res. 2017 Jan-Mar;771:99-127. doi: 10.1016/j.mrrev.2017.02.001. Epub 2017 Feb 16. Mutat Res Rev Mutat Res. 2017. PMID: 28342455 Free PMC article. Review.
-
HiIDDD: a high-throughput imaging pipeline for the quantitative detection of DNA damage in primary human immune cells.Sci Rep. 2022 Apr 15;12(1):6335. doi: 10.1038/s41598-022-10018-0. Sci Rep. 2022. PMID: 35428779 Free PMC article.
References
-
- Abbotts R, Madhusudan S. Human AP endonuclease 1 (APE1): From mechanistic insights to druggable target in cancer. Cancer Treatment Reviews. 2010;36:425–435. - PubMed
-
- Bandaru V, Sunkara S, Wallace SS, Bond JP. A novel human DNA gly-cosylase that removes oxidative DNA damage and is homologous to Escherichia coli endonuclease VIII. DNA Repair (Amsterdam) 2002;1:517–529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

