Activated prostaglandin D2 receptors on macrophages enhance neutrophil recruitment into the lung
- PMID: 26792210
- PMCID: PMC4954606
- DOI: 10.1016/j.jaci.2015.11.012
Activated prostaglandin D2 receptors on macrophages enhance neutrophil recruitment into the lung
Abstract
Background: Prostaglandin (PG) D2 is an early-phase mediator in inflammation, but its action and the roles of the 2 D-type prostanoid receptors (DPs) DP1 and DP2 (also called chemoattractant receptor-homologous molecule expressed on T(H)2 cells) in regulating macrophages have not been elucidated to date.
Objective: We investigated the role of PGD2 receptors on primary human macrophages, as well as primary murine lung macrophages, and their ability to influence neutrophil action in vitro and in vivo.
Methods: In vitro studies, including migration, Ca(2+) flux, and cytokine secretion, were conducted with primary human monocyte-derived macrophages and neutrophils and freshly isolated murine alveolar and pulmonary interstitial macrophages. In vivo pulmonary inflammation was assessed in male BALB/c mice.
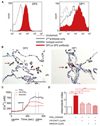
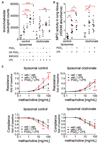
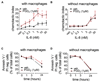
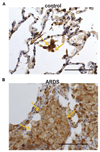
Results: Activation of DP1, DP2, or both receptors on human macrophages induced strong intracellular Ca(2+) flux, cytokine release, and migration of macrophages. In a murine model of LPS-induced pulmonary inflammation, activation of each PGD2 receptor resulted in aggravated airway neutrophilia, tissue myeloperoxidase activity, cytokine contents, and decreased lung compliance. Selective depletion of alveolar macrophages abolished the PGD2-enhanced inflammatory response. Activation of PGD2 receptors on human macrophages enhanced the migratory capacity and prolonged the survival of neutrophils in vitro. In human lung tissue specimens both DP1 and DP2 receptors were located on alveolar macrophages along with hematopoietic PGD synthase, the rate-limiting enzyme of PGD2 synthesis.
Conclusion: For the first time, our results show that PGD2 markedly augments disease activity through its ability to enhance the proinflammatory actions of macrophages and subsequent neutrophil activation.
Keywords: D-type prostanoid receptor 1; D-type prostanoid receptor 2/chemoattractant receptor–homologous molecule expressed on T(H)2 cells; hematopoietic prostaglandin D synthase; macrophages; neutrophils; prostaglandin D(2); pulmonary inflammation.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure of potential conflict of interest: E. M. Sturm has received research support from the Austrian National Bank (grant #14446). I. Aringer is employed by the Medical University of Graz. V. Konya has received research support from Austrian Science Fund FWF (P25531-B23) and from the European Union’s Horizon 2020 (Marie Sklodowska-Curie grant 655677). S.-E. Dahlen has received research support from the Swedish MRC, Heart-Lung Foundations, and many other foundations; is a board member for, has received consultancy fees from, and has received lecture fees from AstraZeneca, Hydra, RSPR Pharma, and Chugai Pharmaceuticals; and has received lecture fees from Novartis and GlaxoSmithKline. R. Schuligoi has received research support from the Austrian Science Fund FWF (P26185-B19). A. Heinemann has received research support from Austrian Science Funds FWF, Austrian National Bank OeNB, AstraZeneca, 7TM Pharma, and Almirall; has received consultancy fees from AstraZeneca; and is a board member for Amgen and Bayer. The rest of the authors declare that they have no relevant conflicts of interest.
Figures


Similar articles
-
Opposing immunomodulatory roles of prostaglandin D2 during the progression of skin inflammation.J Immunol. 2014 Jan 1;192(1):459-65. doi: 10.4049/jimmunol.1302080. Epub 2013 Dec 2. J Immunol. 2014. PMID: 24298012
-
Differential modulation of human basophil functions through prostaglandin D2 receptors DP and chemoattractant receptor-homologous molecule expressed on Th2 cells/DP2.Clin Exp Allergy. 2004 Aug;34(8):1283-90. doi: 10.1111/j.1365-2222.2004.02027.x. Clin Exp Allergy. 2004. PMID: 15298571
-
Prostaglandin D2 causes preferential induction of proinflammatory Th2 cytokine production through an action on chemoattractant receptor-like molecule expressed on Th2 cells.J Immunol. 2005 Nov 15;175(10):6531-6. doi: 10.4049/jimmunol.175.10.6531. J Immunol. 2005. PMID: 16272307
-
The prostaglandin D2 receptor 2 pathway in asthma: a key player in airway inflammation.Respir Res. 2018 Sep 29;19(1):189. doi: 10.1186/s12931-018-0893-x. Respir Res. 2018. PMID: 30268119 Free PMC article. Review.
-
Discovery of anti-inflammatory role of prostaglandin D2.J Vet Med Sci. 2016 Dec 1;78(11):1643-1647. doi: 10.1292/jvms.16-0347. Epub 2016 Aug 5. J Vet Med Sci. 2016. PMID: 27498997 Free PMC article. Review.
Cited by
-
Low oxygen levels decrease adaptive immune responses and ameliorate experimental asthma in mice.Allergy. 2022 Mar;77(3):870-882. doi: 10.1111/all.15020. Epub 2021 Aug 1. Allergy. 2022. PMID: 34309864 Free PMC article.
-
Endogenous PGD2 acting on DP2 receptor counter regulates Schistosoma mansoni infection-driven hepatic granulomatous fibrosis.PLoS Pathog. 2024 Aug 22;20(8):e1011812. doi: 10.1371/journal.ppat.1011812. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39173086 Free PMC article.
-
CRTH2 in Pulmonary Fibrosis: Friend or Foe?Am J Respir Cell Mol Biol. 2022 Aug;67(2):145-146. doi: 10.1165/rcmb.2022-0232ED. Am J Respir Cell Mol Biol. 2022. PMID: 35675551 Free PMC article. No abstract available.
-
Non-eosinophilic asthma in nonsteroidal anti-inflammatory drug exacerbated respiratory disease.Clin Transl Allergy. 2023 Mar;13(3):e12235. doi: 10.1002/clt2.12235. Clin Transl Allergy. 2023. PMID: 36973957 Free PMC article.
-
Basement membrane product, endostatin, as a link between inflammation, coagulation and vascular permeability in COVID-19 and non-COVID-19 acute respiratory distress syndrome.Front Immunol. 2023 May 22;14:1188079. doi: 10.3389/fimmu.2023.1188079. eCollection 2023. Front Immunol. 2023. PMID: 37283766 Free PMC article.
References
-
- Schuligoi R, Sturm E, Luschnig P, Konya V, Philipose S, Sedej M, et al. CRTH2 and D-type prostanoid receptor antagonists as novel therapeutic agents for inflammatory diseases. Pharmacology. 2010;85:372–82. - PubMed
-
- Liston TE, Roberts LJ. Transformation of prostaglandin D2 to 9 alpha, 11 beta-(15S)-trihydroxyprosta-(5Z,13E)-dien-1-oic acid (9 alpha, 11 beta-prostaglandin F2): a unique biologically active prostaglandin produced enzymatically in vivo in humans. Proc Natl Acad Sci U S A. 1985;82:6030–4. - PMC - PubMed
-
- Shiraishi Y, Asano K, Nakajima T, Oguma T, Suzuki Y, Shiomi T, et al. Prostaglandin D2-induced eosinophilic airway inflammation is mediated by CRTH2 receptor. J Pharmacol Exp Ther. 2005;312:954–60. - PubMed
-
- Spik I, Brénuchon C, Angéli V, Staumont D, Fleury S, Capron M, et al. Activation of the prostaglandin D2 receptor DP2/CRTH2 increases allergic inflammation in mouse. J Immunol. 2005;174:3703–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

