Contribution of elastic tissues to the mechanics and energetics of muscle function during movement
- PMID: 26792339
- PMCID: PMC6514471
- DOI: 10.1242/jeb.124446
Contribution of elastic tissues to the mechanics and energetics of muscle function during movement
Abstract
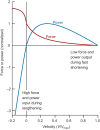
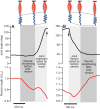
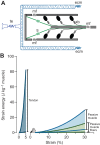
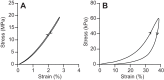
Muscle force production occurs within an environment of tissues that exhibit spring-like behavior, and this elasticity is a critical determinant of muscle performance during locomotion. Muscle force and power output both depend on the speed of contraction, as described by the isotonic force-velocity curve. By influencing the speed of contractile elements, elastic structures can have a profound effect on muscle force, power and work. In very rapid movements, elastic mechanisms can amplify muscle power by storing the work of muscle contraction slowly and releasing it rapidly. When energy must be dissipated rapidly, such as in landing from a jump, energy stored rapidly in elastic elements can be released more slowly to stretch muscle contractile elements, reducing the power input to muscle and possibly protecting it from damage. Elastic mechanisms identified so far rely primarily on in-series tendons, but many structures within muscles exhibit spring-like properties. Actomyosin cross-bridges, actin and myosin filaments, titin, and the connective tissue scaffolding of the extracellular matrix all have the potential to store and recover elastic energy during muscle contraction. The potential contribution of these elements can be assessed from their stiffness and estimates of the strain they undergo during muscle function. Such calculations provide boundaries for the possible roles these springs might play in locomotion, and may help to direct future studies of the uses of elastic elements in muscle.
Keywords: Elastic energy; Locomotion; Metabolic economy; Tendon.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures
Similar articles
-
The integrated function of muscles and tendons during locomotion.Comp Biochem Physiol A Mol Integr Physiol. 2002 Dec;133(4):1087-99. doi: 10.1016/s1095-6433(02)00244-1. Comp Biochem Physiol A Mol Integr Physiol. 2002. PMID: 12485693
-
Flexible mechanisms: the diverse roles of biological springs in vertebrate movement.J Exp Biol. 2011 Feb 1;214(Pt 3):353-61. doi: 10.1242/jeb.038588. J Exp Biol. 2011. PMID: 21228194 Free PMC article. Review.
-
Exploiting elasticity: Modeling the influence of neural control on mechanics and energetics of ankle muscle-tendons during human hopping.J Theor Biol. 2014 Jul 21;353:121-32. doi: 10.1016/j.jtbi.2014.03.010. Epub 2014 Mar 16. J Theor Biol. 2014. PMID: 24641822
-
Storage and recovery of elastic potential energy powers ballistic prey capture in toads.J Exp Biol. 2006 Jul;209(Pt 13):2535-53. doi: 10.1242/jeb.02276. J Exp Biol. 2006. PMID: 16788037
-
Muscle-tendon unit design and tuning for power enhancement, power attenuation, and reduction of metabolic cost.J Biomech. 2023 May;153:111585. doi: 10.1016/j.jbiomech.2023.111585. Epub 2023 Apr 13. J Biomech. 2023. PMID: 37126884 Free PMC article. Review.
Cited by
-
Altered Protein Composition and Gene Expression in Strabismic Human Extraocular Muscles and Tendons.Invest Ophthalmol Vis Sci. 2016 Oct 1;57(13):5576-5585. doi: 10.1167/iovs.16-20294. Invest Ophthalmol Vis Sci. 2016. PMID: 27768799 Free PMC article.
-
Research progress in pelvic floor ultrasound for assessing the morphology and function of levator ani muscle in women.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023 Aug 28;48(8):1267-1273. doi: 10.11817/j.issn.1672-7347.2023.220577. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023. PMID: 37875368 Free PMC article. Chinese, English.
-
Profile of 50 m Sprinting: The Influence of Carbon-Plated Spikes on Maximum-Velocity Performance.Sensors (Basel). 2025 Mar 22;25(7):1979. doi: 10.3390/s25071979. Sensors (Basel). 2025. PMID: 40218490 Free PMC article.
-
Ultrasound shear wave seeds reduced following hamstring strain injury but not after returning to sport.Insights Imaging. 2024 Jan 8;15(1):7. doi: 10.1186/s13244-023-01571-x. Insights Imaging. 2024. PMID: 38191955 Free PMC article.
-
Despite Good Correlations, There Is No Exact Coincidence between Isometric and Dynamic Strength Measurements in Elite Youth Soccer Players.Sports (Basel). 2022 Nov 10;10(11):175. doi: 10.3390/sports10110175. Sports (Basel). 2022. PMID: 36355825 Free PMC article.
References
-
- Aerts P. (1997). Vertical jumping in Galago senegalensis: the quest for an obligate mechanical power amplifier. Philos. Trans. R. Soc. B Biol. Sci. 353, 1607-1620. 10.1098/rstb.1998.0313 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

