Locomotion as an emergent property of muscle contractile dynamics
- PMID: 26792341
- PMCID: PMC6514473
- DOI: 10.1242/jeb.123935
Locomotion as an emergent property of muscle contractile dynamics
Abstract
Skeletal muscles share many common, highly conserved features of organization at the molecular and myofilament levels, giving skeletal muscle fibers generally similar and characteristic mechanical and energetic properties; properties well described by classical studies of muscle mechanics and energetics. However, skeletal muscles can differ considerably in architectural design (fiber length, pinnation, and connective tissue organization), as well as fiber type, and how they contract in relation to the timing of neuromotor activation and in vivo length change. The in vivo dynamics of muscle contraction is, therefore, crucial to assessing muscle design and the roles that muscles play in animal movement. Architectural differences in muscle-tendon organization combined with differences in the phase of activation and resulting fiber length changes greatly affect the time-varying force and work that muscles produce, as well as the energetic cost of force generation. Intrinsic force-length and force-velocity properties of muscles, together with their architecture, also play important roles in the control of movement, facilitating rapid adjustments to changing motor demands. Such adjustments complement slower, reflex-mediated neural feedback control of motor recruitment. Understanding how individual fiber forces are integrated to whole-muscle forces, which are transmitted to the skeleton for producing and controlling locomotor movement, is therefore essential for assessing muscle design in relation to the dynamics of movement.
Keywords: Fascicle strain; In vivo contraction; Muscle activation; Muscle-tendon architecture; Muscle-tendon force.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The author declares no competing or financial interests.
Figures


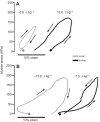

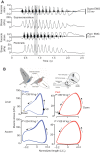
References
-
- Biewener A. A. (1998a). Muscle function in vivo: the design of muscles used as springs versus muscles used to generate mechanical power. Am. Zool. 38, 703-717. 10.1093/icb/38.4.703 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

