Wasted research when systematic reviews fail to provide a complete and up-to-date evidence synthesis: the example of lung cancer
- PMID: 26792360
- PMCID: PMC4719540
- DOI: 10.1186/s12916-016-0555-0
Wasted research when systematic reviews fail to provide a complete and up-to-date evidence synthesis: the example of lung cancer
Abstract
Background: Multiple treatments are frequently available for a given condition, and clinicians and patients need a comprehensive, up-to-date synthesis of evidence for all competing treatments. We aimed to quantify the waste of research related to the failure of systematic reviews to provide a complete and up-to-date evidence synthesis over time.
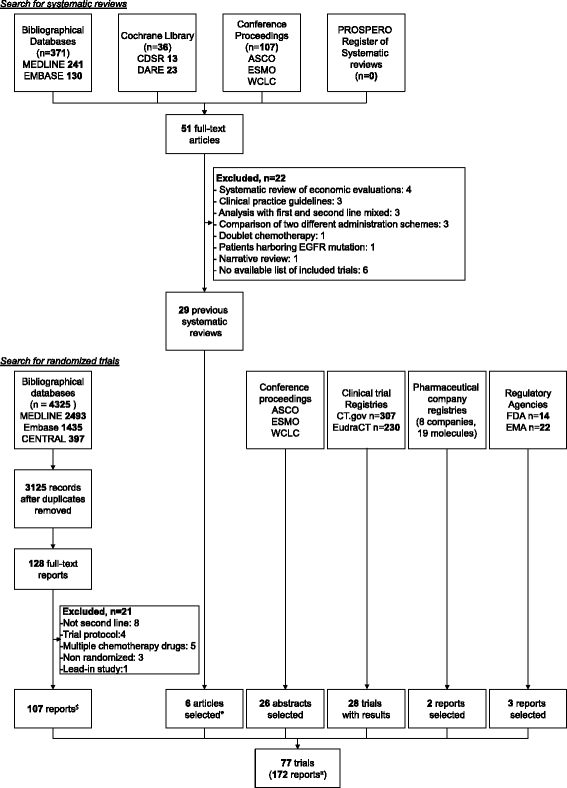
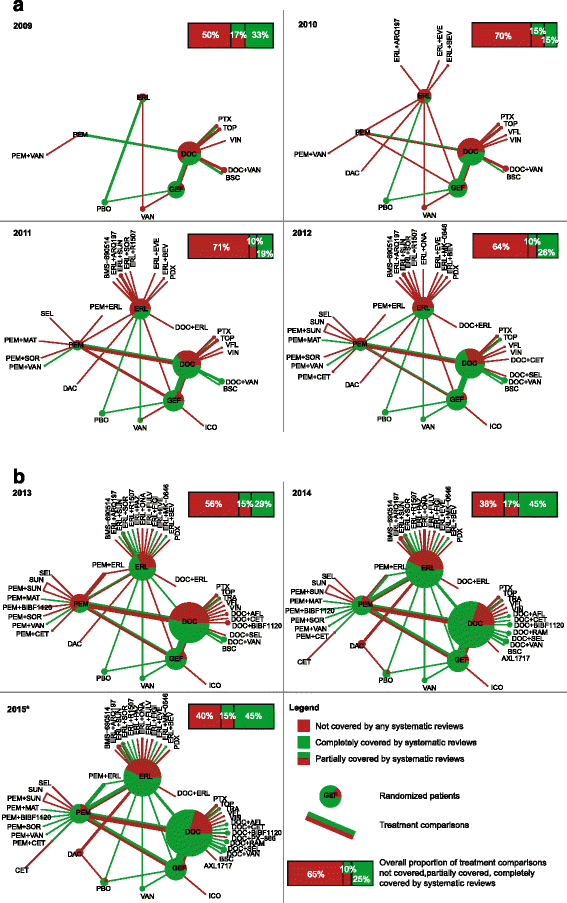
Methods: We performed a series of systematic overviews and networks of randomized trials assessing the gap between evidence covered by systematic reviews and available trials of second-line treatments for advanced non-small cell lung cancer. We searched the Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, MEDLINE, EMBASE, and other resources sequentially by year from 2009 to March 2, 2015. We sequentially compared the amount of evidence missing from systematic reviews to the randomized evidence available for inclusion each year. We constructed cumulative networks of randomized evidence over time and evaluated the proportion of trials, patients, treatments, and treatment comparisons not covered by systematic reviews on December 31 each year from 2009 to 2015.
Results: We identified 77 trials (28,636 patients) assessing 47 treatments with 54 comparisons and 29 systematic reviews (13 published after 2013). From 2009 to 2015, the evidence covered by existing systematic reviews was consistently incomplete: 45 % to 70 % of trials; 30 % to 58 % of patients; 40 % to 66 % of treatments; and 38 % to 71 % of comparisons were missing. In the cumulative networks of randomized evidence, 10 % to 17 % of treatment comparisons were partially covered by systematic reviews and 55 % to 85 % were partially or not covered.
Conclusions: We illustrate how systematic reviews of a given condition provide a fragmented, out-of-date panorama of the evidence for all treatments. This waste of research might be reduced by the development of live cumulative network meta-analyses.
Figures



Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Bias due to selective inclusion and reporting of outcomes and analyses in systematic reviews of randomised trials of healthcare interventions.Cochrane Database Syst Rev. 2014 Oct 1;2014(10):MR000035. doi: 10.1002/14651858.MR000035.pub2. Cochrane Database Syst Rev. 2014. PMID: 25271098 Free PMC article.
-
Interventions for escalation of therapy for acute exacerbations of asthma in children: an overview of Cochrane Reviews.Cochrane Database Syst Rev. 2020 Aug 5;8(8):CD012977. doi: 10.1002/14651858.CD012977.pub2. Cochrane Database Syst Rev. 2020. PMID: 32767571 Free PMC article.
-
Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
-
Effectiveness and safety of chemotherapy with cytokine-induced killer cells in non-small cell lung cancer: A systematic review and meta-analysis of 32 randomized controlled trials.Cytotherapy. 2019 Feb;21(2):125-147. doi: 10.1016/j.jcyt.2018.10.011. Epub 2018 Dec 14. Cytotherapy. 2019. PMID: 30554868
Cited by
-
Therapeutic options for advanced epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer: a Bayesian network secondary analysis.Aging (Albany NY). 2020 Apr 23;12(8):7129-7162. doi: 10.18632/aging.103066. Epub 2020 Apr 23. Aging (Albany NY). 2020. PMID: 32324592 Free PMC article.
-
A pilot validation study of crowdsourcing systematic reviews: update of a searchable database of pediatric clinical trials of high-dose vitamin D.Transl Pediatr. 2017 Jan;6(1):18-26. doi: 10.21037/tp.2016.12.01. Transl Pediatr. 2017. PMID: 28164026 Free PMC article.
-
Living cumulative network meta-analysis to reduce waste in research: A paradigmatic shift for systematic reviews?BMC Med. 2016 Mar 29;14:59. doi: 10.1186/s12916-016-0596-4. BMC Med. 2016. PMID: 27025849 Free PMC article.
-
Living network meta-analysis compared with pairwise meta-analysis in comparative effectiveness research: empirical study.BMJ. 2018 Feb 28;360:k585. doi: 10.1136/bmj.k585. BMJ. 2018. PMID: 29490922 Free PMC article. Review.
-
Comparative efficacy and safety of second-line treatments for advanced non-small cell lung cancer with wild-type or unknown status for epidermal growth factor receptor: a systematic review and network meta-analysis.BMC Med. 2017 Oct 30;15(1):193. doi: 10.1186/s12916-017-0954-x. BMC Med. 2017. PMID: 29082855 Free PMC article.
References
-
- Institute of Medicine . Initial national priorities for comparative effectiveness research. Washington, DC: The National Academies Press; 2009.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

