Fluoroquinolone interactions with Mycobacterium tuberculosis gyrase: Enhancing drug activity against wild-type and resistant gyrase
- PMID: 26792518
- PMCID: PMC4763725
- DOI: 10.1073/pnas.1525055113
Fluoroquinolone interactions with Mycobacterium tuberculosis gyrase: Enhancing drug activity against wild-type and resistant gyrase
Abstract
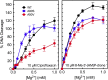
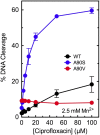
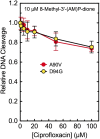
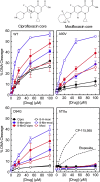
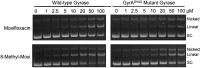
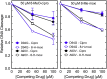
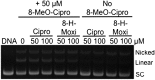
Mycobacterium tuberculosis is a significant source of global morbidity and mortality. Moxifloxacin and other fluoroquinolones are important therapeutic agents for the treatment of tuberculosis, particularly multidrug-resistant infections. To guide the development of new quinolone-based agents, it is critical to understand the basis of drug action against M. tuberculosis gyrase and how mutations in the enzyme cause resistance. Therefore, we characterized interactions of fluoroquinolones and related drugs with WT gyrase and enzymes carrying mutations at GyrA(A90) and GyrA(D94). M. tuberculosis gyrase lacks a conserved serine that anchors a water-metal ion bridge that is critical for quinolone interactions with other bacterial type II topoisomerases. Despite the fact that the serine is replaced by an alanine (i.e., GyrA(A90)) in M. tuberculosis gyrase, the bridge still forms and plays a functional role in mediating quinolone-gyrase interactions. Clinically relevant mutations at GyrA(A90) and GyrA(D94) cause quinolone resistance by disrupting the bridge-enzyme interaction, thereby decreasing drug affinity. Fluoroquinolone activity against WT and resistant enzymes is enhanced by the introduction of specific groups at the C7 and C8 positions. By dissecting fluoroquinolone-enzyme interactions, we determined that an 8-methyl-moxifloxacin derivative induces high levels of stable cleavage complexes with WT gyrase and two common resistant enzymes, GyrA(A90V) and GyrA(D94G). 8-Methyl-moxifloxacin was more potent than moxifloxacin against WT M. tuberculosis gyrase and displayed higher activity against the mutant enzymes than moxifloxacin did against WT gyrase. This chemical biology approach to defining drug-enzyme interactions has the potential to identify novel drugs with improved activity against tuberculosis.
Keywords: Mycobacterium tuberculosis; antibiotic resistance; complex stability; fluoroquinolones; gyrase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- World Health Organization Tuberculosis. Fact Sheet No 104. 2014 www.who.int/mediacentre/factsheets/fs104/en/Accessed July 21, 2014.
-
- World Health Organization . Treatment of Tuberculosis: Guidelines. 4th Ed WHO Press; Geneva: 2010. - PubMed
-
- TB CARE I . International Standards for Tuberculosis Care. 2014. , Edition 3 (TB CARE I, The Hague)
-
- American Thoracic Society CDC Infectious Diseases Society of America Treatment of tuberculosis. MMWR Recomm Rep. 2003;52(RR-11):1–77, and erratum (2005) 53:1203. - PubMed
-
- World Health Organization . Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis. WHO Press; Geneva: 2011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

