Genistein exposure inhibits growth and alters steroidogenesis in adult mouse antral follicles
- PMID: 26792615
- PMCID: PMC4744102
- DOI: 10.1016/j.taap.2015.12.026
Genistein exposure inhibits growth and alters steroidogenesis in adult mouse antral follicles
Abstract
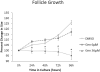
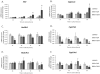
Genistein is a naturally occurring isoflavone phytoestrogen commonly found in plant products such as soybeans, lentils, and chickpeas. Genistein, like other phytoestrogens, has the potential to mimic, enhance, or impair the estradiol biosynthesis pathway, thereby potentially altering ovarian follicle growth. Previous studies have inconsistently indicated that genistein exposure may alter granulosa cell proliferation and hormone production, but no studies have examined the effects of genistein on intact antral follicles. Thus, this study was designed to test the hypothesis that genistein exposure inhibits follicle growth and steroidogenesis in intact antral follicles. To test this hypothesis, antral follicles isolated from CD-1 mice were cultured with vehicle (dimethyl sulfoxide; DMSO) or genistein (6.0 and 36μM) for 18-96h. Every 24h, follicle diameters were measured to assess growth. At the end of each culture period, the media were pooled to measure hormone levels, and the cultured follicles were collected to measure expression of cell cycle regulators and steroidogenic enzymes. The results indicate that genistein (36μM) inhibits growth of mouse antral follicles. Additionally, genistein (6.0 and 36μM) increases progesterone, testosterone, and dehydroepiandrosterone (DHEA) levels, but decreases estrone and estradiol levels. The results also indicate that genistein alters the expression of steroidogenic enzymes at 24, 72 and 96h, and the expression of cell cycle regulators at 18h. These data indicate that genistein exposure inhibits antral follicle growth by inhibiting the cell cycle, alters sex steroid hormone levels, and dysregulates steroidogenic enzymes in cultured mouse antral follicles.
Keywords: Follicle; Genistein; Ovary; Steroidogenesis.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
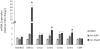
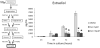
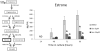
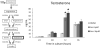
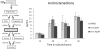
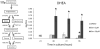
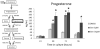
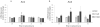
Similar articles
-
Effects of isoliquiritigenin on ovarian antral follicle growth and steroidogenesis.Reprod Toxicol. 2016 Dec;66:107-114. doi: 10.1016/j.reprotox.2016.10.004. Epub 2016 Oct 20. Reprod Toxicol. 2016. PMID: 27773742 Free PMC article.
-
Di(2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles.Toxicol Appl Pharmacol. 2015 Apr 1;284(1):42-53. doi: 10.1016/j.taap.2015.02.010. Epub 2015 Feb 18. Toxicol Appl Pharmacol. 2015. PMID: 25701202 Free PMC article.
-
Equol inhibits growth, induces atresia, and inhibits steroidogenesis of mouse antral follicles in vitro.Toxicol Appl Pharmacol. 2016 Mar 15;295:47-55. doi: 10.1016/j.taap.2016.02.009. Epub 2016 Feb 11. Toxicol Appl Pharmacol. 2016. PMID: 26876617 Free PMC article.
-
[Human folliculogenesis and local regulation].Nihon Sanka Fujinka Gakkai Zasshi. 1995 Aug;47(8):726-37. Nihon Sanka Fujinka Gakkai Zasshi. 1995. PMID: 7594882 Review. Japanese.
-
Physiological effects and mechanisms of action of endocrine disrupting chemicals that alter estrogen signaling.Hormones (Athens). 2010 Jul-Sep;9(3):191-205. doi: 10.14310/horm.2002.1270. Hormones (Athens). 2010. PMID: 20688617 Free PMC article. Review. No abstract available.
Cited by
-
In vitro ovarian follicle growth: a comprehensive analysis of key protocol variables†.Biol Reprod. 2020 Aug 21;103(3):455-470. doi: 10.1093/biolre/ioaa073. Biol Reprod. 2020. PMID: 32406908 Free PMC article. Review.
-
Enhancement of Productive Performance, Bone Physical Characteristics, and Mineralization of Laying Hens during the Post-Peak Period by Genistein.Arch Razi Inst. 2021 Jul;76(2):359-369. doi: 10.22092/ari.2020.342143.1454. Epub 2021 Jul 1. Arch Razi Inst. 2021. PMID: 34223734 Free PMC article.
-
The Antioxidant Activity of Atomized Extracts of the Leaves and Stems of Cnidoscolus diacanthus (Pax & K. Hoffm.) J.F. Macbr. from Peru and Their Effect on Sex Hormone Levels in Rats.Molecules. 2024 Sep 25;29(19):4554. doi: 10.3390/molecules29194554. Molecules. 2024. PMID: 39407486 Free PMC article.
-
Genistein improves the reproductive performance and bone status of breeder hens during the late egg-laying period.Poult Sci. 2019 Dec 1;98(12):7022-7029. doi: 10.3382/ps/pez367. Poult Sci. 2019. PMID: 31309232 Free PMC article.
-
Preconception exposure to dietary levels of genistein affects female reproductive outcomes.Reprod Toxicol. 2017 Dec;74:174-180. doi: 10.1016/j.reprotox.2017.09.014. Epub 2017 Sep 29. Reprod Toxicol. 2017. PMID: 28970133 Free PMC article.
References
-
- Andres S, Abraham K, Appel KE, Lampen A. Risks and benefits of dietary isoflavones for cancer. Critical reviews in toxicology. 2011;41:463–506. - PubMed
-
- Armstrong DT. Gonadotropins, ovarian metabolism, and steroid biosynthesis. Recent progress in hormone research. 1968;24:255–319. - PubMed
-
- Bagur AC, Mautalen CA. Risk for developing osteoporosis in untreated premature menopause. Calcified tissue international. 1992;51:4–7. - PubMed
-
- Basini G, Bussolati S, Santini SE, Grasselli F. The impact of the phyto-oestrogen genistein on swine granulosa cell function. Journal of animal physiology and animal nutrition. 2010;94:e374–382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

