Conditional Deletion of Murine Fgf23: Interruption of the Normal Skeletal Responses to Phosphate Challenge and Rescue of Genetic Hypophosphatemia
- PMID: 26792657
- PMCID: PMC4891276
- DOI: 10.1002/jbmr.2792
Conditional Deletion of Murine Fgf23: Interruption of the Normal Skeletal Responses to Phosphate Challenge and Rescue of Genetic Hypophosphatemia
Abstract
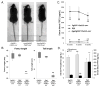
The transgenic and knockout (KO) animals involving Fgf23 have been highly informative in defining novel aspects of mineral metabolism, but are limited by shortened lifespan, inability of spatial/temporal FGF23 control, and infertility of the global KO. To more finely test the role of systemic and genetic influences in FGF23 production, a mouse was developed that carried a floxed ("f")-Fgf23 allele (exon 2 floxed) which demonstrated in vivo recombination when bred to global-Cre transgenic mice (eIIa-cre). Mice homozygous for the recombined allele ("Δ") had undetectable serum intact FGF23, elevated serum phosphate (p < 0.05), and increased kidney Cyp27b1 mRNA (p < 0.05), similar to global Fgf23-KO mice. To isolate cellular FGF23 responses during phosphate challenge, Fgf23(Δ/f) mice were mated with early osteoblast type Iα1 collagen 2.3-kb promoter-cre mice (Col2.3-cre) and the late osteoblast/early osteocyte Dentin matrix protein-1-cre (Dmp1-cre). Fgf23(Δ/f) /Col2.3-cre(+) and Fgf23(Δ/f) /Dmp1-cre(+) exhibited reduced baseline serum intact FGF23 versus controls. After challenge with high-phosphate diet Cre(-) mice had 2.1-fold to 2.5-fold increased serum FGF23 (p < 0.01), but Col2.3-cre(+) mice had no significant increase, and Dmp1-cre(+) mice had only a 37% increase (p < 0.01) despite prevailing hyperphosphatemia in both models. The Fgf23(Δ/f) /Col2.3-cre was bred onto the Hyp (murine X-linked hypophosphatemia [XLH] model) genetic background to test the contribution of osteoblasts and osteocytes to elevated FGF23 and Hyp disease phenotypes. Whereas Hyp mice maintained inappropriately elevated FGF23 considering their marked hypophosphatemia, Hyp/Fgf23(Δ/f) /Col2.3-cre(+) mice had serum FGF23 <4% of Hyp (p < 0.01), and this targeted restriction normalized serum phosphorus and ricketic bone disease. In summary, deleting FGF23 within early osteoblasts and osteocytes demonstrated that both cell types contribute to baseline circulating FGF23 concentrations, and that targeting osteoblasts/osteocytes for FGF23 production can modify systemic responses to changes in serum phosphate concentrations and rescue the Hyp genetic syndrome. © 2016 American Society for Bone and Mineral Research.
Keywords: CRE-RECOMBINASE; FGF-23; KLOTHO; OSTEOBLAST; OSTEOCYTE; PHOSPHATE; VITAMIN D; XLH.
© 2016 American Society for Bone and Mineral Research.
Conflict of interest statement
The other authors have no conflicts.
Figures

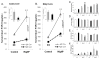

Similar articles
-
Osteocyte-specific deletion of Fgfr1 suppresses FGF23.PLoS One. 2014 Aug 4;9(8):e104154. doi: 10.1371/journal.pone.0104154. eCollection 2014. PLoS One. 2014. PMID: 25089825 Free PMC article.
-
Aberrant Phex function in osteoblasts and osteocytes alone underlies murine X-linked hypophosphatemia.J Clin Invest. 2008 Feb;118(2):722-34. doi: 10.1172/JCI32702. J Clin Invest. 2008. PMID: 18172553 Free PMC article.
-
Dysregulated gene expression in the primary osteoblasts and osteocytes isolated from hypophosphatemic Hyp mice.PLoS One. 2014 Apr 7;9(4):e93840. doi: 10.1371/journal.pone.0093840. eCollection 2014. PLoS One. 2014. PMID: 24710520 Free PMC article.
-
Regulation of bone-renal mineral and energy metabolism: the PHEX, FGF23, DMP1, MEPE ASARM pathway.Crit Rev Eukaryot Gene Expr. 2012;22(1):61-86. doi: 10.1615/critreveukargeneexpr.v22.i1.50. Crit Rev Eukaryot Gene Expr. 2012. PMID: 22339660 Free PMC article. Review.
-
Roles of osteocytes in phosphate metabolism.Front Endocrinol (Lausanne). 2022 Jul 15;13:967774. doi: 10.3389/fendo.2022.967774. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35909535 Free PMC article. Review.
Cited by
-
Osteocytic FGF23 and Its Kidney Function.Front Endocrinol (Lausanne). 2020 Aug 28;11:592. doi: 10.3389/fendo.2020.00592. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32982979 Free PMC article. Review.
-
FGF23 and its role in X-linked hypophosphatemia-related morbidity.Orphanet J Rare Dis. 2019 Feb 26;14(1):58. doi: 10.1186/s13023-019-1014-8. Orphanet J Rare Dis. 2019. PMID: 30808384 Free PMC article. Review.
-
Increased FGF23 protects against detrimental cardio-renal consequences during elevated blood phosphate in CKD.JCI Insight. 2019 Feb 21;4(4):e123817. doi: 10.1172/jci.insight.123817. eCollection 2019 Feb 21. JCI Insight. 2019. PMID: 30830862 Free PMC article.
-
In Vivo Analysis of the Contribution of Proprotein Convertases to the Processing of FGF23.Front Endocrinol (Lausanne). 2021 Jun 4;12:690681. doi: 10.3389/fendo.2021.690681. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34149625 Free PMC article.
-
Hypoxia Pathway Proteins are Master Regulators of Erythropoiesis.Int J Mol Sci. 2020 Oct 30;21(21):8131. doi: 10.3390/ijms21218131. Int J Mol Sci. 2020. PMID: 33143240 Free PMC article. Review.
References
-
- Tenenhouse HS, Econs MJ. In: The Metabolic and Molecular Bases of Inherited Disease; Valle D, editor. New York: The McGraw-Hill Companies; 2001. pp. 1–9.
-
- White KE, Larsson TE, Econs MJ. The roles of specific genes implicated as circulating factors involved in normal and disordered phosphate homeostasis: frizzled related protein-4, matrix extracellular phosphoglycoprotein, and fibroblast growth factor 23. Endocr Rev. 2006;27(3):221–41. - PubMed
-
- Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444(7120):770–4. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

