A two years open-label prospective study of OnabotulinumtoxinA 195 U in medication overuse headache: a real-world experience
- PMID: 26792662
- PMCID: PMC4720620
- DOI: 10.1186/s10194-016-0591-3
A two years open-label prospective study of OnabotulinumtoxinA 195 U in medication overuse headache: a real-world experience
Abstract
Background: The efficacy and safety of OnabotulinumtoxinA (BOTOX®) in adults with chronic migraine (CM) were demonstrated in the PREEMPT program. However, the dosage used in this study was flexible from 155 U to 195 U at the physician's discretion. Therefore, the objective of this prospective study was to compare the efficacy and safety of OnabotulinumtoxinA 195 U vs. 155 U for the treatment of CM and medication overuse headache (MOH) during a 2-year period.
Methods: We prospectively evaluated the mean reduction in headache days, migraine days, acute pain medication intake days and Headache Impact Test (HIT)-6 score in 172 patients injected with OnabotulinumtoxinA 195 U. Successively, we compared the efficacy measures with data of 155 patients injected with OnabotulinumtoxinA 155 U and followed up for 2 years. All patients were affected by CM and MOH, and failed one or more previous detoxification and preventative therapies.
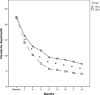
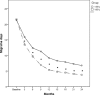
Results: Both OnabotulinumtoxinA 195 U and 155 U reduced significantly the number of headache and migraine days, acute pain medication intake days and HIT-6 score, when compared with the baseline measures. Nevertheless, OnabotulinumtoxinA 195 U proved to be superior of 155 U in all efficacy measures since the first injection and for all the 2 years of treatment, with the exception of the reduction in pain medication intake days that resulted significantly larger with 195 U only after the 4th injection. The safety and tolerability of the two doses were similar and treatment related adverse events were transient and mild-moderate.
Conclusions: This study represents the largest and longest post-marketing studies of doses comparison with OnabotulinumtoxinA in a real-life clinical setting. Here, we demonstrate the superior efficacy of OnabotulinumtoxinA 195 U compared to 155 U in CM patients with MOH during a 2-year treatment period with similar safety and tolerability profile.
Keywords: Chronic migraine; Medication overuse headache; Migraine abuse; OnabotulinumtoxinA; Preventative therapy.
Figures


Similar articles
-
An open-label prospective study of the real-life use of onabotulinumtoxinA for the treatment of chronic migraine: the REPOSE study.J Headache Pain. 2019 Mar 7;20(1):26. doi: 10.1186/s10194-019-0976-1. J Headache Pain. 2019. PMID: 30845917 Free PMC article.
-
OnabotulinumtoxinA 155 U in medication overuse headache: a two years prospective study.Springerplus. 2015 Dec 30;4:826. doi: 10.1186/s40064-015-1636-9. eCollection 2015. Springerplus. 2015. PMID: 26753113 Free PMC article.
-
Long-term study of the efficacy and safety of OnabotulinumtoxinA for the prevention of chronic migraine: COMPEL study.J Headache Pain. 2018 Feb 5;19(1):13. doi: 10.1186/s10194-018-0840-8. J Headache Pain. 2018. PMID: 29404713 Free PMC article. Clinical Trial.
-
Development of onabotulinumtoxinA for chronic migraine.Ann N Y Acad Sci. 2014 Nov;1329:67-80. doi: 10.1111/nyas.12488. Epub 2014 Aug 18. Ann N Y Acad Sci. 2014. PMID: 25399521 Review.
-
Chronic migraine treatment: from OnabotulinumtoxinA onwards.Expert Rev Neurother. 2016 Oct;16(10):1217-27. doi: 10.1080/14737175.2016.1200973. Epub 2016 Jul 4. Expert Rev Neurother. 2016. PMID: 27310178 Review.
Cited by
-
Is prednisone still a reasonable option in the treatment of withdrawal headache in patients with chronic migraine and medication overuse headache in the age of CGRP antibodies? A narrative review.Headache. 2022 Nov;62(10):1264-1271. doi: 10.1111/head.14415. Epub 2022 Nov 27. Headache. 2022. PMID: 36437611 Free PMC article. Review.
-
The Use of Botulinum Toxin in the Management of Headache Disorders.Handb Exp Pharmacol. 2021;263:227-249. doi: 10.1007/164_2020_365. Handb Exp Pharmacol. 2021. PMID: 32562057 Clinical Trial.
-
Cognitive effects of onabotulinumtoxinA in chronic migraine.BMJ Neurol Open. 2020 Jan 9;2(1):e000014. doi: 10.1136/bmjno-2019-000014. eCollection 2020. BMJ Neurol Open. 2020. PMID: 33681771 Free PMC article.
-
Onabotulinum toxin A in the treatment of chronic migraine: patient selection and special considerations.J Pain Res. 2017 Sep 29;10:2319-2329. doi: 10.2147/JPR.S113614. eCollection 2017. J Pain Res. 2017. PMID: 29033605 Free PMC article. Review.
-
Excellent Response to OnabotulinumtoxinA: Different Definitions, Different Predictors.Int J Environ Res Public Health. 2022 Sep 2;19(17):10975. doi: 10.3390/ijerph191710975. Int J Environ Res Public Health. 2022. PMID: 36078699 Free PMC article.
References
-
- Global Burden of Disease Study 2013 Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. - DOI - PMC - PubMed
-
- Natoli JL, Manack A, Dean B, Butler Q, Turkel CC, Stovner L, Lipton RB. Global prevalence of chronic migraine: a systematic review. Cephalalgia. 2010;30:599–609. - PubMed
-
- Blumenfeld AM, Varon SF, Wilcox TK, Buse DC, Kawata AK, Manack A, Goadsby PJ, Lipton RB. Disability, HRQoL and resource use among chronic and episodic migraineurs: Results from the International Burden of Migraine Study (IBMS) Cephalalgia. 2011;31:301–315. doi: 10.1177/0333102410381145. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

