Possible role of Krüppel-like factor 5 in the remodeling of small airways and pulmonary vessels in chronic obstructive pulmonary disease
- PMID: 26792671
- PMCID: PMC4719583
- DOI: 10.1186/s12931-016-0322-y
Possible role of Krüppel-like factor 5 in the remodeling of small airways and pulmonary vessels in chronic obstructive pulmonary disease
Abstract
Background: Small airway remodeling is an important cause of the airflow limitation in chronic obstructive pulmonary disease (COPD). A large population of patients with COPD also have pulmonary hypertension. Krüppel-like factor 5 (KLF5) is a zinc-finger transcription factor that contributes to tissue remodeling in cardiovascular diseases. Here, we evaluate the possible involvement of KLF5 in the remodeling of small airways and pulmonary vessels in COPD.
Methods: Lung tissues were obtained from 23 control never-smokers, 17 control ex-smokers and 24 ex-smokers with COPD. The expression of KLF5 in the lung tissues was investigated by immunohistochemistry. We investigated whether oxidative/nitrosative stress, which is a major cause of the pathogenesis in COPD, could augment the production of KLF5. We examined the role of KLF5 in the stress-mediated tissue remodeling responses. We also investigated the susceptibility of KLF5 expression to nitrosative stress using bronchial fibroblasts isolated from the lung tissues.
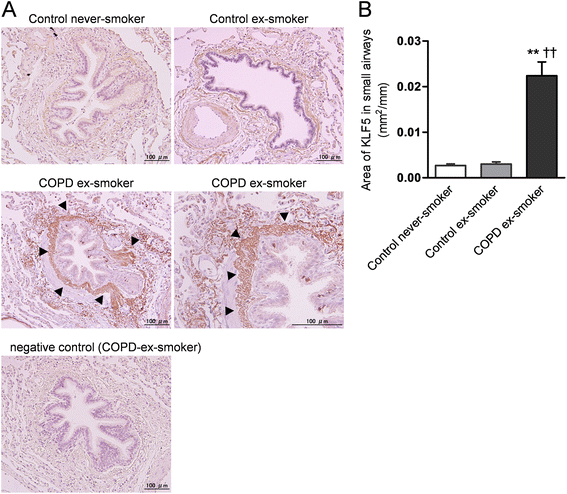
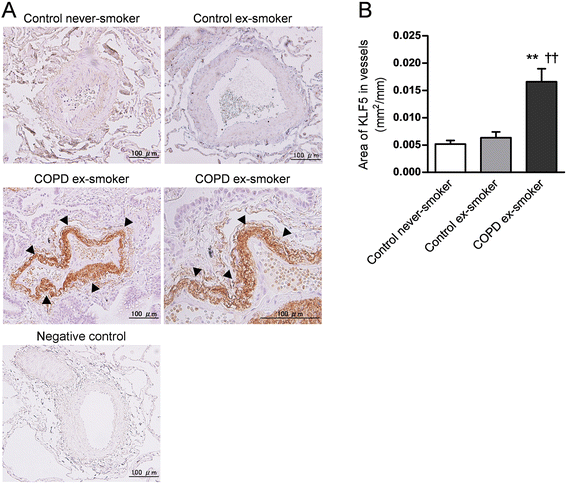
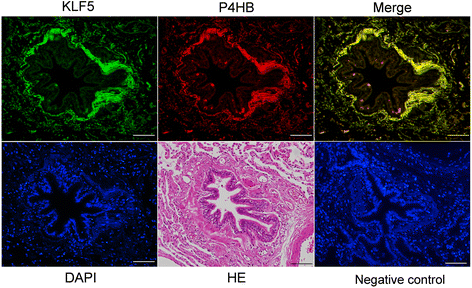
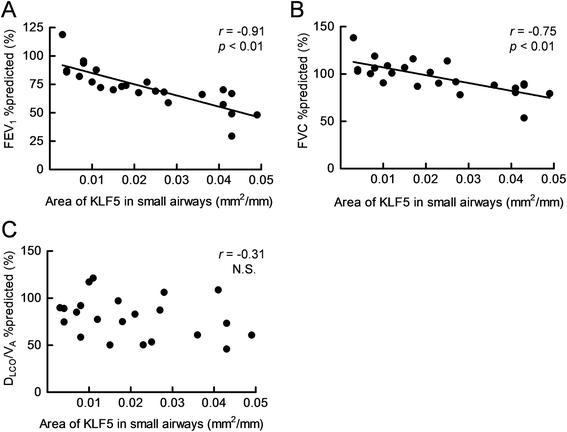
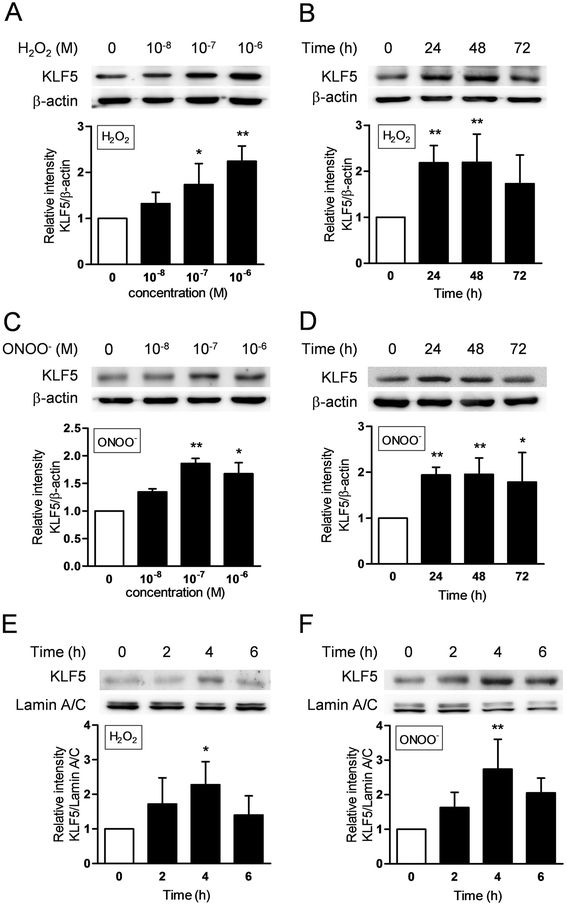
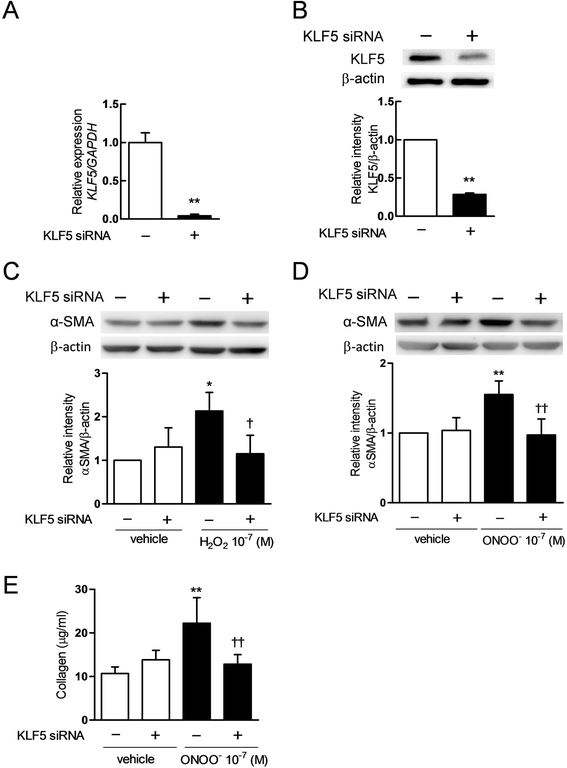
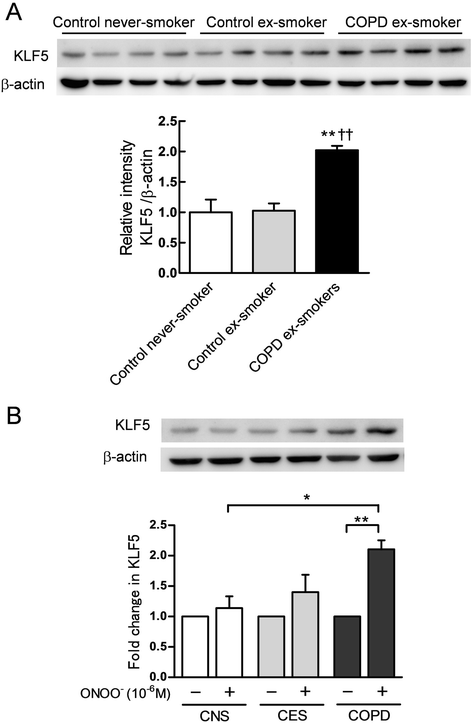
Results: The expression of KLF5 was up-regulated in the small airways and pulmonary vessels of the COPD patients and it was mainly expressed in bronchial fibroblasts and cells of the pulmonary vessels. The extent of the KLF5 expression in the small airway of the COPD group had a significant correlation with the severity of the airflow limitation. Oxidative/nitrosative stress augmented the production of KLF5 in lung fibroblasts as well as the translocation of KLF5 into the nuclei. Silencing of KLF5 suppressed the stress-augmented differentiation into myofibroblasts, the release of collagens and metalloproteinases. Bronchial fibroblasts from the patients with COPD highly expressed KLF5 compared to those from the control subjects under basal condition and were more susceptible to the induction of KLF5 expression by nitrosative stress compared to those from the control subjects.
Conclusion: We provide the first evidence that the expression of KLF5 is up-regulated in small airways and pulmonary vessels of patients with COPD and may be involved in the tissue remodeling of COPD.
Figures

Similar articles
-
[Hypoxia induced the remodeling of pulmonary arterial smooth muscles and increased the pulmonary artery smooth muscle Krüppel-like zinc-finger transcription factor 5 expression].Zhonghua Jie He He Hu Xi Za Zhi. 2016 Oct 12;39(10):791-795. doi: 10.3760/cma.j.issn.1001-0939.2016.10.010. Zhonghua Jie He He Hu Xi Za Zhi. 2016. PMID: 27784498 Chinese.
-
miR-145-5p is associated with smoke-related chronic obstructive pulmonary disease via targeting KLF5.Chem Biol Interact. 2019 Feb 25;300:82-90. doi: 10.1016/j.cbi.2019.01.011. Epub 2019 Jan 9. Chem Biol Interact. 2019. PMID: 30639269
-
Increased expression of TROP2 in airway basal cells potentially contributes to airway remodeling in chronic obstructive pulmonary disease.Respir Res. 2016 Nov 25;17(1):159. doi: 10.1186/s12931-016-0463-z. Respir Res. 2016. PMID: 27887617 Free PMC article.
-
Remodeling in asthma and COPD--differences and similarities.Clin Respir J. 2010 May;4 Suppl 1:20-7. doi: 10.1111/j.1752-699X.2010.00193.x. Clin Respir J. 2010. PMID: 20500606 Review.
-
Pulmonary vascular changes in asthma and COPD.Pulm Pharmacol Ther. 2014 Dec;29(2):144-55. doi: 10.1016/j.pupt.2014.09.003. Epub 2014 Oct 12. Pulm Pharmacol Ther. 2014. PMID: 25316209 Review.
Cited by
-
Hyperinsulinemia-induced KLF5 mediates endothelial angiogenic dysfunction in diabetic endothelial cells.J Mol Histol. 2019 Jun;50(3):239-251. doi: 10.1007/s10735-019-09821-3. Epub 2019 May 2. J Mol Histol. 2019. PMID: 31049798
-
Genome-wide association study of asthma, total IgE, and lung function in a cohort of Peruvian children.J Allergy Clin Immunol. 2021 Dec;148(6):1493-1504. doi: 10.1016/j.jaci.2021.02.035. Epub 2021 Mar 10. J Allergy Clin Immunol. 2021. PMID: 33713768 Free PMC article.
-
Roles of Krüppel-like factor 5 in kidney disease.J Cell Mol Med. 2021 Mar;25(5):2342-2355. doi: 10.1111/jcmm.16332. Epub 2021 Feb 1. J Cell Mol Med. 2021. PMID: 33523554 Free PMC article. Review.
-
Small airway dysfunction in pneumoconiosis: a cross-sectional study.BMC Pulm Med. 2022 Apr 28;22(1):167. doi: 10.1186/s12890-022-01929-9. BMC Pulm Med. 2022. PMID: 35484546 Free PMC article.
-
Down-regulation of KLF5 in cancer-associated fibroblasts inhibit gastric cancer cells progression by CCL5/CCR5 axis.Cancer Biol Ther. 2017 Oct 3;18(10):806-815. doi: 10.1080/15384047.2017.1373219. Epub 2017 Sep 21. Cancer Biol Ther. 2017. PMID: 28934010 Free PMC article.
References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2014. Global Strategy for the Diagnosis, Management and Prevention of COPD. www.goldcopd.org/uploads/users/files/GOLD_Report_ Report_2015_Apr2.pdf. Date last updated: January 2015. Date last accessed: August 11 2015.
-
- Takizawa H, Tanaka M, Takami K, Ohtoshi T, Ito K, Satoh M, et al. Increased expression of transforming growth factor-beta1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD) Am J Respir Crit Care Med. 2001;163:1476–83. doi: 10.1164/ajrccm.163.6.9908135. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

