Dissecting the Molecular Mechanisms of the Tropism of Varicella-Zoster Virus for Human T Cells
- PMID: 26792747
- PMCID: PMC4794656
- DOI: 10.1128/JVI.03375-14
Dissecting the Molecular Mechanisms of the Tropism of Varicella-Zoster Virus for Human T Cells
Abstract
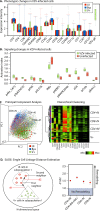
Studies of varicella-zoster virus (VZV) tropism for T cells support their role in viral transport to the skin during primary infection. Multiparametric single-cell mass cytometry demonstrates that, instead of preferentially infecting skin-homing T cells, VZV alters cell signaling and remodels surface proteins to enhance T cell skin trafficking. Viral proteins dispensable in skin, such as that encoded by open reading frame 66, are necessary in T cells. Interference with VZV T cell tropism may offer novel strategies for drug and vaccine design.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

