Epithelial Control of Gut-Associated Lymphoid Tissue Formation through p38α-Dependent Restraint of NF-κB Signaling
- PMID: 26792803
- PMCID: PMC4761524
- DOI: 10.4049/jimmunol.1501724
Epithelial Control of Gut-Associated Lymphoid Tissue Formation through p38α-Dependent Restraint of NF-κB Signaling
Abstract
The protein kinase p38α mediates cellular responses to environmental and endogenous cues that direct tissue homeostasis and immune responses. Studies of mice lacking p38α in several different cell types have demonstrated that p38α signaling is essential to maintaining the proliferation-differentiation balance in developing and steady-state tissues. The mechanisms underlying these roles involve cell-autonomous control of signaling and gene expression by p38α. In this study, we show that p38α regulates gut-associated lymphoid tissue (GALT) formation in a noncell-autonomous manner. From an investigation of mice with intestinal epithelial cell-specific deletion of the p38α gene, we find that p38α serves to limit NF-κB signaling and thereby attenuate GALT-promoting chemokine expression in the intestinal epithelium. Loss of this regulation results in GALT hyperplasia and, in some animals, mucosa-associated B cell lymphoma. These anomalies occur independently of luminal microbial stimuli and are most likely driven by direct epithelial-lymphoid interactions. Our study illustrates a novel p38α-dependent mechanism preventing excessive generation of epithelial-derived signals that drive lymphoid tissue overgrowth and malignancy.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures
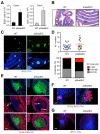
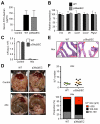
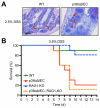

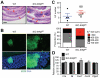

References
-
- Brewster JL, Gustin MC. Hog1: 20 years of discovery and impact. Sci. Signal. 2014;7:re7. - PubMed
-
- Trempolec N, Dave-Coll N, Nebreda AR. SnapShot: p38 MAPK signaling. Cell. 2013;152:656–656.e1. - PubMed
-
- Hui L, Bakiri L, Mairhorfer A, Schweifer N, Haslinger C, Kenner L, Komnenovic V, Scheuch H, Beug H, Wagner EF. p38α suppresses normal and cancer cell proliferation by antagonizing the JNK-c-Jun pathway. Nat. Genet. 2007;39:741–749. - PubMed
-
- Ventura JJ, Tenbaum S, Perdiguero E, Huth M, Guerra C, Barbacid M, Pasparakis M, Nebreda AR. p38α MAP kinase is essential in lung stem and progenitor cell proliferation and differentiation. Nat. Genet. 2007;39:750–758. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

