Deficiency of the alkaline ceramidase ACER3 manifests in early childhood by progressive leukodystrophy
- PMID: 26792856
- PMCID: PMC5068917
- DOI: 10.1136/jmedgenet-2015-103457
Deficiency of the alkaline ceramidase ACER3 manifests in early childhood by progressive leukodystrophy
Abstract
Background/aims: Leukodystrophies due to abnormal production of myelin cause extensive morbidity in early life; their genetic background is still largely unknown. We aimed at reaching a molecular diagnosis in Ashkenazi-Jewish patients who suffered from developmental regression at 6-13 months, leukodystrophy and peripheral neuropathy.
Methods: Exome analysis, determination of alkaline ceramidase activity catalysing the conversion of C18:1-ceramide to sphingosine and D-ribo-C12-N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) (NBD)-phytoceramide to NBD-C12-fatty acid using liquid chromatography-tandem mass spectrometry (LC-MS/MS) and thin layer chromatography, respectively, and sphingolipid analysis in patients' blood by LC-MS/MS.
Results: The patients were homozygous for p.E33G in the ACER3, which encodes a C18:1-alkaline ceramidase and C20:1-alkaline ceramidase. The mutation abolished ACER3 catalytic activity in the patients' cells and failed to restore alkaline ceramidase activity in yeast mutant strain. The levels of ACER3 substrates, C18:1-ceramides and dihydroceramides and C20:1-ceramides and dihydroceramides and other long-chain ceramides and dihydroceramides were markedly increased in the patients' plasma, along with that of complex sphingolipids, including monohexosylceramides and lactosylceramides.
Conclusions: Homozygosity for the p.E33G mutation in the ACER3 gene results in inactivation of ACER3, leading to the accumulation of various sphingolipids in blood and probably in brain, likely accounting for this new form of childhood leukodystrophy.
Keywords: Neurology.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Conflict of interest statement
Conflicts of Interest: None declared.
Figures


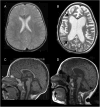
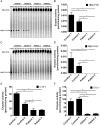
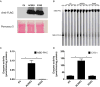


References
-
- Mao C, Xu R, Bielawska A, Obeid LM. Cloning of an alkaline ceramidase from Saccharomyces cerevisiae. An enzyme with reverse (CoA-independent) ceramide synthase activity. J Biol Chem. 2000;275:6876–84. - PubMed
-
- Mao C, Xu R, Szulc ZM, Bielawska A, Galadari SH, Obeid LM. Cloning and characterization of a novel human alkaline ceramidase. A mammalian enzyme that hydrolyzes phytoceramide. J Biol Chem. 2001;276:26577–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
