Selective Increase of Auditory Cortico-Striatal Coherence during Auditory-Cued Go/NoGo Discrimination Learning
- PMID: 26793085
- PMCID: PMC4707278
- DOI: 10.3389/fnbeh.2015.00368
Selective Increase of Auditory Cortico-Striatal Coherence during Auditory-Cued Go/NoGo Discrimination Learning
Abstract
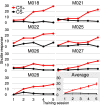
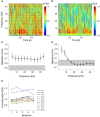
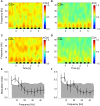
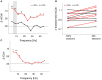
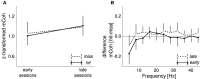
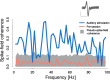
Goal directed behavior and associated learning processes are tightly linked to neuronal activity in the ventral striatum. Mechanisms that integrate task relevant sensory information into striatal processing during decision making and learning are implicitly assumed in current reinforcement models, yet they are still weakly understood. To identify the functional activation of cortico-striatal subpopulations of connections during auditory discrimination learning, we trained Mongolian gerbils in a two-way active avoidance task in a shuttlebox to discriminate between falling and rising frequency modulated tones with identical spectral properties. We assessed functional coupling by analyzing the field-field coherence between the auditory cortex and the ventral striatum of animals performing the task. During the course of training, we observed a selective increase of functional coupling during Go-stimulus presentations. These results suggest that the auditory cortex functionally interacts with the ventral striatum during auditory learning and that the strengthening of these functional connections is selectively goal-directed.
Keywords: Mongolian gerbil; auditory cortex; avoidance learning; discrimination learning; field-field coherence; functional coupling; shuttlebox; ventral striatum.
Figures
References
-
- Barth D. S., Di S. (1990). Three-dimensional analysis of auditory-evoked potentials in rat neocortex. J. Neurophysiol. 64, 1527–1536. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

