CYP2D7 Sequence Variation Interferes with TaqMan CYP2D6 (*) 15 and (*) 35 Genotyping
- PMID: 26793106
- PMCID: PMC4709848
- DOI: 10.3389/fphar.2015.00312
CYP2D7 Sequence Variation Interferes with TaqMan CYP2D6 (*) 15 and (*) 35 Genotyping
Abstract
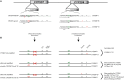
TaqMan™ genotyping assays are widely used to genotype CYP2D6, which encodes a major drug metabolizing enzyme. Assay design for CYP2D6 can be challenging owing to the presence of two pseudogenes, CYP2D7 and CYP2D8, structural and copy number variation and numerous single nucleotide polymorphisms (SNPs) some of which reflect the wild-type sequence of the CYP2D7 pseudogene. The aim of this study was to identify the mechanism causing false-positive CYP2D6 (*) 15 calls and remediate those by redesigning and validating alternative TaqMan genotype assays. Among 13,866 DNA samples genotyped by the CompanionDx® lab on the OpenArray platform, 70 samples were identified as heterozygotes for 137Tins, the key SNP of CYP2D6 (*) 15. However, only 15 samples were confirmed when tested with the Luminex xTAG CYP2D6 Kit and sequencing of CYP2D6-specific long range (XL)-PCR products. Genotype and gene resequencing of CYP2D6 and CYP2D7-specific XL-PCR products revealed a CC>GT dinucleotide SNP in exon 1 of CYP2D7 that reverts the sequence to CYP2D6 and allows a TaqMan assay PCR primer to bind. Because CYP2D7 also carries a Tins, a false-positive mutation signal is generated. This CYP2D7 SNP was also responsible for generating false-positive signals for rs769258 (CYP2D6 (*) 35) which is also located in exon 1. Although alternative CYP2D6 (*) 15 and (*) 35 assays resolved the issue, we discovered a novel CYP2D6 (*) 15 subvariant in one sample that carries additional SNPs preventing detection with the alternate assay. The frequency of CYP2D6 (*) 15 was 0.1% in this ethnically diverse U.S. population sample. In addition, we also discovered linkage between the CYP2D7 CC>GT dinucleotide SNP and the 77G>A (rs28371696) SNP of CYP2D6 (*) 43. The frequency of this tentatively functional allele was 0.2%. Taken together, these findings emphasize that regardless of how careful genotyping assays are designed and evaluated before being commercially marketed, rare or unknown SNPs underneath primer and/or probe regions can impact the performance of PCR-based genotype assays, including TaqMan. Regardless of the test platform used, it is prudent to confirm rare allele calls by an independent method.
Keywords: CYP2D6; CYP2D6*15; CYP2D6*35; CYP2D6*43; CYP2D7; TaqMan; genotyping.
Figures



References
-
- Alcazar-González G. A., Calderon-Garciduenas A. L., Garza-Rodriguez M. L., Rubio-Hernandez G., Escorza-Trevino S., Olano-Martin E., et al. . (2013). Comparative study of polymorphism frequencies of the CYP2D6, CYP3A5, CYP2C8 and IL-10 genes in Mexican and Spanish women with breast cancer. Pharmacogenomics 14, 1583–1592. 10.2217/pgs.13.83 - DOI - PubMed
-
- Crews K. R., Gaedigk A., Dunnenberger H. M., Leeder J. S., Klein T. E., Caudle K. E., et al. (2014). Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for cytochrome P450 2D6 (CYP2D6) genotype and codeine therapy: 2014 Update. Clin. Pharmacol. Ther. 95, 376–382. 10.1038/clpt.2013.254 - DOI - PMC - PubMed
-
- Dunnenberger H. M., Crews K. R., Hoffman J. M., Caudle K. E., Broeckel U., Howard S. C., et al. . (2015). Preemptive clinical pharmacogenetics implementation: current programs in five US medical centers. Annu. Rev. Pharmacol. Toxicol. 55, 89–106. 10.1146/annurev-pharmtox-010814-124835 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

