Circadian Mechanisms Underlying Reward-Related Neurophysiology and Synaptic Plasticity
- PMID: 26793129
- PMCID: PMC4709415
- DOI: 10.3389/fpsyt.2015.00187
Circadian Mechanisms Underlying Reward-Related Neurophysiology and Synaptic Plasticity
Abstract
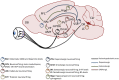
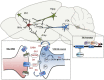
Evidence from clinical and preclinical research provides an undeniable link between disruptions in the circadian clock and the development of psychiatric diseases, including mood and substance abuse disorders. The molecular clock, which controls daily patterns of physiological and behavioral activity in living organisms, when desynchronized, may exacerbate or precipitate symptoms of psychiatric illness. One of the outstanding questions remaining in this field is that of cause and effect in the relationship between circadian rhythm disruption and psychiatric disease. Focus has recently turned to uncovering the role of circadian proteins beyond the maintenance of homeostatic systems and outside of the suprachiasmatic nucleus (SCN), the master pacemaker region of the brain. In this regard, several groups, including our own, have sought to understand how circadian proteins regulate mechanisms of synaptic plasticity and neurotransmitter signaling in mesocorticolimbic brain regions, which are known to be critically involved in reward processing and mood. This regulation can come in the form of direct transcriptional control of genes central to mood and reward, including those associated with dopaminergic activity in the midbrain. It can also be seen at the circuit level through indirect connections of mesocorticolimbic regions with the SCN. Circadian misalignment paradigms as well as genetic models of circadian disruption have helped to elucidate some of the complex interactions between these systems and neural activity influencing behavior. In this review, we explore findings that link circadian protein function with synaptic adaptations underlying plasticity as it may contribute to the development of mood disorders and addiction. In light of recent advances in technology and sophisticated methods for molecular and circuit-level interrogation, we propose future directions aimed at teasing apart mechanisms through which the circadian system modulates mood and reward-related behavior.
Keywords: circadian; mouse models; psychiatric disorders; reward; synaptic transmission and plasticity.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

