Function and Dynamics of Tetraspanins during Antigen Recognition and Immunological Synapse Formation
- PMID: 26793193
- PMCID: PMC4707441
- DOI: 10.3389/fimmu.2015.00653
Function and Dynamics of Tetraspanins during Antigen Recognition and Immunological Synapse Formation
Abstract
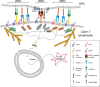
Tetraspanin-enriched microdomains (TEMs) are specialized membrane platforms driven by protein-protein interactions that integrate membrane receptors and adhesion molecules. Tetraspanins participate in antigen recognition and presentation by antigen--presenting cells (APCs) through the organization of pattern-recognition receptors (PRRs) and their downstream-induced signaling, as well as the regulation of MHC-II-peptide trafficking. T lymphocyte activation is triggered upon specific recognition of antigens present on the APC surface during immunological synapse (IS) formation. This dynamic process is characterized by a defined spatial organization involving the compartmentalization of receptors and adhesion molecules in specialized membrane domains that are connected to the underlying cytoskeleton and signaling molecules. Tetraspanins contribute to the spatial organization and maturation of the IS by controlling receptor clustering and local accumulation of adhesion receptors and integrins, their downstream signaling, and linkage to the actin cytoskeleton. This review offers a perspective on the important role of TEMs in the regulation of antigen recognition and presentation and in the dynamics of IS architectural organization.
Keywords: T-cell activation; adhesion receptors; immunological synapse; tetraspanin-enriched microdomains; tetraspanins.
Figures

References
-
- Berditchevski F, Odintsova E, Sawada S, Gilbert E. Expression of the palmitoylation-deficient CD151 weakens the association of alpha 3 beta 1 integrin with the tetraspanin-enriched microdomains and affects integrin-dependent signaling. J Biol Chem (2002) 277:36991–7000.10.1074/jbc.M205265200 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

