Copper Delivery to Chloroplast Proteins and its Regulation
- PMID: 26793223
- PMCID: PMC4709454
- DOI: 10.3389/fpls.2015.01250
Copper Delivery to Chloroplast Proteins and its Regulation
Abstract
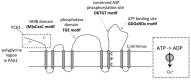
Copper is required for photosynthesis in chloroplasts of plants because it is a cofactor of plastocyanin, an essential electron carrier in the thylakoid lumen. Other chloroplast copper proteins are copper/zinc superoxide dismutase and polyphenol oxidase, but these proteins seem to be dispensable under conditions of low copper supply when transcripts for these proteins undergo microRNA-mediated down regulation. Two ATP-driven copper transporters function in tandem to deliver copper to chloroplast compartments. This review seeks to summarize the mechanisms of copper delivery to chloroplast proteins and its regulation. We also delineate some of the unanswered questions that still remain in this field.
Keywords: Cu-miRNA; copper deficiency; copper transporting P-type ATPase; photosynthesis; plastocyanin; polyphenol oxidase; superoxide dismutase.
Figures


References
-
- Abdel-Ghany S. E., Burkhead J. L., Gogolin K. A., Andres-Colas N., Bodecker J. R., Puig S., et al. (2005b). AtCCS is a functional homolog of the yeast copper chaperone Ccs1/Lys7. FEBS Lett. 579 2307–2312. - PubMed
-
- Andres-Colas N., Sancenon V., Rodriguez-Navarro S., Mayo S., Thiele D. J., Ecker J. R., et al. (2006). The Arabidopsis heavy metal P-type ATPase HMA5 interacts with metallochaperones and functions in copper detoxification of roots. Plant J. 45 225–236. 10.1111/j.1365-313X.2005.02601.x - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

