Comparative Physiological and Transcriptional Analyses of Two Contrasting Drought Tolerant Alfalfa Varieties
- PMID: 26793226
- PMCID: PMC4709457
- DOI: 10.3389/fpls.2015.01256
Comparative Physiological and Transcriptional Analyses of Two Contrasting Drought Tolerant Alfalfa Varieties
Abstract
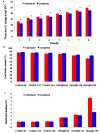
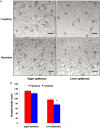
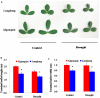
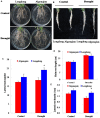
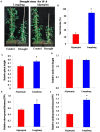
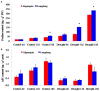
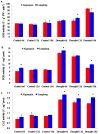
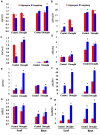
Drought is one of major environmental determinants of plant growth and productivity. Alfalfa (Medicago sativa) is a legume perennial forage crop native to the arid and semi-arid environment, which is an ideal candidate to study the biochemical and molecular mechanisms conferring drought resistance in plants. In this study, drought stress responses of two alfalfa varieties, Longdong and Algonquin, were comparatively assayed at the physiological, morphological, and transcriptional levels. Under control condition, the drought-tolerant Longdong with smaller leaf size and lower stomata density showed less water loss than the drought-sensitive Algonquin. After exposing to drought stress, Longdong showed less severe cell membrane damage, more proline, and ascorbate (ASC) contents and less accumulation of H2O2 than Algonquin. Moreover, significantly higher antioxidant enzymes activities after drought treatment were found in Longdong when compared with Algonquin. In addition, transcriptional expression analysis showed that Longdong exhibited significantly higher transcripts of drought-responsive genes in leaf and root under drought stress condition. Taken together, these results indicated that Longdong variety was more drought-tolerant than Algonquin variety as evidenced by less leaf firing, more lateral root number, higher relative aboveground/underground biomass per plant and survival rate.
Keywords: alfalfa; drought stress; lateral root; physiological changes; stomata density; transcriptional expression.
Figures

References
-
- Ashraf M., Foolad M. (2007). Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 59 206–216. 10.1016/j.envexpbot.2005.12.006 - DOI
-
- Bao A. K., Du B. Q., Touil L., Kang P., Wang Q. L., Wang S. M. (2015). Co-expression of tonoplast Cation/H+ antiporter and H+-pyrophosphatase from xerophyte Zygophyllum xanthoxylum improves alfalfa plant growth under salinity, drought and field conditions. Plant Biotechnol. J. 10.1111/pbi.12451 [Epub ahead of print]. - DOI - PMC - PubMed
-
- Castroluna A., Ruiz O. M., Quiroga A. M. (2014). Effects of salinity and drought stress on germination, biomass and growth in three varieties of Medicago sativa L. Avances Invest. Agropec. 18 39–50.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

