Anticoagulation management in mechanical circulatory support
- PMID: 26793333
- PMCID: PMC4703699
- DOI: 10.3978/j.issn.2072-1439.2015.10.65
Anticoagulation management in mechanical circulatory support
Abstract
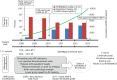
Heart failure continues to be a worldwide epidemic, effecting over 23 million persons. Despite advances in medical therapy, the disease is progressive and a significant proportion of patients will need advanced heart replacement therapy. Continuous flow assist devices have become a standard approach for many patients both as a bridge to cardiac transplantation and as destination therapy (DT). However, device related complications such as bleeding and thrombosis continue to hinder further advancements of this technology. The field is rapidly advancing and efforts to reduce pump complications are directed towards improving hemocompatibility and maximizing blood flow without clinically significant hemolysis, areas of stasis or turbulent flow.
Keywords: Anticoagulation; acquired von Willebrand syndrome; anti-factor Xa (anti-FXa); continuous-flow left ventricular assist device (CF-LVAD); device thrombosis; mean arterial pressure; mechanical circulatory support.
Conflict of interest statement
Figures


References
-
- Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med 2014;370:33-40. - PubMed
-
- Kirklin JK, Naftel DC, Kormos RL, et al. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. J Heart Lung Transplant 2014;33:12-22. - PubMed
-
- Najjar SS, Slaughter MS, Pagani FD, et al. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant 2014;33:23-34. - PubMed
-
- Pagani FD, Milano CA, Tatooles AJ, et al. HeartWare HVAD for the Treatment of Patients With Advanced Heart Failure Ineligible for Cardiac Transplantation: Results of the ENDURANCE Destination Therapy Trial. ISHLT 35th Annual Meeting. Nice, France, 2015.
-
- Food and Drug Administration. Serious Adverse Events with Implantable Left Ventricular Assist Devices (LVADs): FDA Safety Communication. Available online: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm457327.htm
Publication types
LinkOut - more resources
Full Text Sources
