In vitro adaptation of Plasmodium falciparum reveal variations in cultivability
- PMID: 26794408
- PMCID: PMC4722725
- DOI: 10.1186/s12936-015-1053-0
In vitro adaptation of Plasmodium falciparum reveal variations in cultivability
Abstract
Background: Culture-adapted Plasmodium falciparum parasites can offer deeper understanding of geographic variations in drug resistance, pathogenesis and immune evasion. To help ground population-based calculations and inferences from culture-adapted parasites, the complete range of parasites from a study area must be well represented in any collection. To this end, standardized adaptation methods and determinants of successful in vitro adaption were sought.
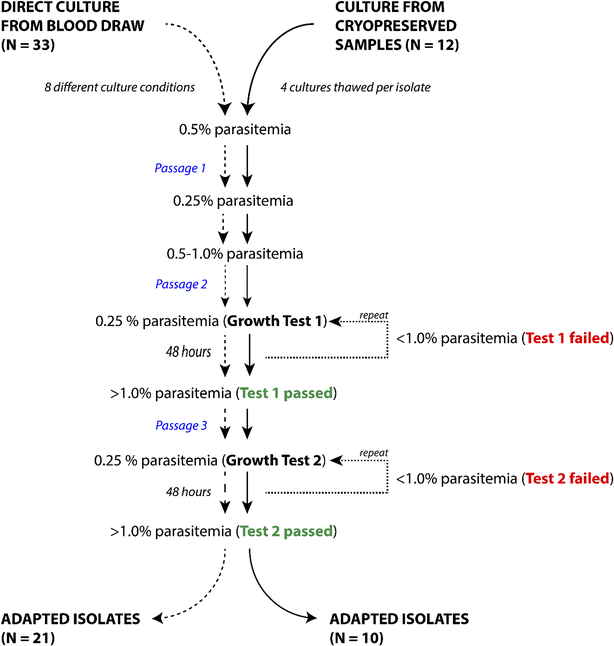
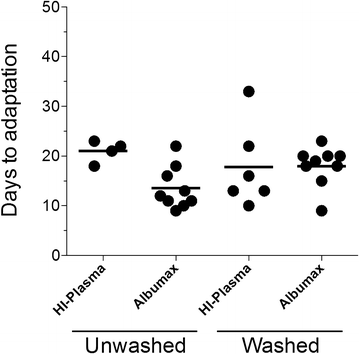
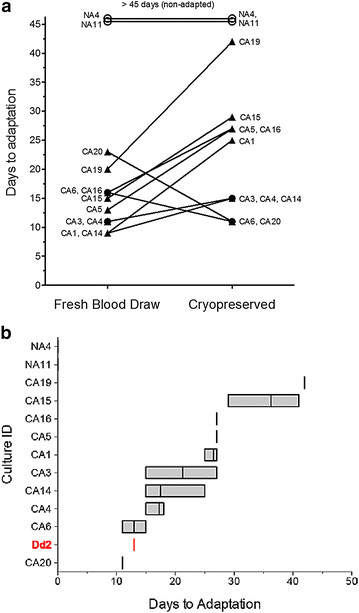
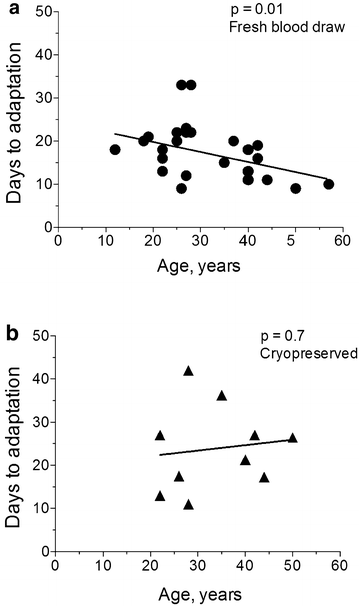
Methods: Venous blood was collected from 33 P. falciparum-infected individuals at Goa Medical College and Hospital (Bambolim, Goa, India). Culture variables such as whole blood versus washed blood, heat-inactivated plasma versus Albumax, and different starting haematocrit levels were tested on fresh blood samples from patients. In vitro adaptation was considered successful when two four-fold or greater increases in parasitaemia were observed within, at most, 33 days of attempted culture. Subsequently, parasites from the same patients, which were originally cryopreserved following blood draw, were retested for adaptability for 45 days using identical host red blood cells (RBCs) and culture media.
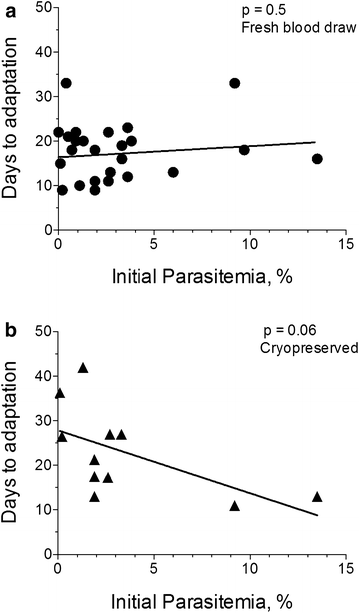
Results: At a new endemic area research site, ~65% of tested patient samples, with varied patient history and clinical presentation, were successfully culture-adapted immediately after blood collection. Cultures set up at 1% haematocrit and 0.5% Albumax adapted most rapidly, but no single test condition was uniformly fatal to culture adaptation. Success was not limited by low patient parasitaemia nor by patient age. Some parasites emerged even after significant delays in sample processing and even after initiation of treatment with anti-malarials. When 'day 0' cryopreserved samples were retested in parallel many months later using identical host RBCs and media, speed to adaptation appeared to be an intrinsic property of the parasites collected from individual patients.
Conclusions: Culture adaptation of P. falciparum in a field setting is formally shown to be robust. Parasites were found to have intrinsic variations in adaptability to culture conditions, with some lines requiring longer attempt periods for successful adaptation. Quantitative approaches described here can help describe phenotypic diversity of field parasite collections with precision. This is expected to improve population-based extrapolations of findings from field-derived fresh culture-adapted parasites to broader questions of public health importance.
Figures
Similar articles
-
The Genotypic and Phenotypic Stability of Plasmodium falciparum Field Isolates in Continuous In Vitro Culture.PLoS One. 2016 Jan 11;11(1):e0143565. doi: 10.1371/journal.pone.0143565. eCollection 2016. PLoS One. 2016. PMID: 26751382 Free PMC article.
-
Short-term cryopreservation and thawing have minimal effects on Plasmodium falciparum ex vivo invasion profile.Front Cell Infect Microbiol. 2022 Sep 20;12:997418. doi: 10.3389/fcimb.2022.997418. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36204649 Free PMC article.
-
[In vitro cultivation of fields isolates of Plasmodium falciparum in Mali].Bull Soc Pathol Exot. 2007 Feb;100(1):3-5. doi: 10.3185/pathexo2883. Bull Soc Pathol Exot. 2007. PMID: 17402683 French.
-
[In vitro cultivation of Plasmodium falciparum. Applications and limits.- Methodology].Med Trop (Mars). 1982 Jul-Aug;42(4):437-62. Med Trop (Mars). 1982. PMID: 6755144 Review. French.
-
Switching Plasmodium falciparum genes on and off for erythrocyte invasion.Trends Parasitol. 2008 Nov;24(11):517-24. doi: 10.1016/j.pt.2008.08.005. Epub 2008 Sep 19. Trends Parasitol. 2008. PMID: 18805736 Review.
Cited by
-
A Novel Ex Vivo Drug Assay for Assessing the Transmission-Blocking Activity of Compounds on Field-Isolated Plasmodium falciparum Gametocytes.Antimicrob Agents Chemother. 2022 Dec 20;66(12):e0100122. doi: 10.1128/aac.01001-22. Epub 2022 Nov 2. Antimicrob Agents Chemother. 2022. PMID: 36321830 Free PMC article.
-
Decreased In Vitro Artemisinin Sensitivity of Plasmodium falciparum across India.Antimicrob Agents Chemother. 2019 Sep 23;63(10):e00101-19. doi: 10.1128/AAC.00101-19. Print 2019 Oct. Antimicrob Agents Chemother. 2019. PMID: 31332065 Free PMC article.
-
Genotypes and phenotypes of resistance in Ecuadorian Plasmodium falciparum.Malar J. 2019 Dec 10;18(1):415. doi: 10.1186/s12936-019-3044-z. Malar J. 2019. PMID: 31822269 Free PMC article.
-
Cultivation of Asexual Intraerythrocytic Stages of Plasmodium falciparum.Pathogens. 2023 Jul 1;12(7):900. doi: 10.3390/pathogens12070900. Pathogens. 2023. PMID: 37513747 Free PMC article. Review.
-
Cholesterol-dependent enrichment of understudied erythrocytic stages of human Plasmodium parasites.Sci Rep. 2020 Mar 12;10(1):4591. doi: 10.1038/s41598-020-61392-6. Sci Rep. 2020. PMID: 32165667 Free PMC article.
References
-
- WHO . World Malaria Report 2014. Geneva: World Health Organization; 2014.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

