Randomized controlled studies on the efficacy of antiarthritic agents in inhibiting cartilage degeneration and pain associated with progression of osteoarthritis in the rat
- PMID: 26794830
- PMCID: PMC4721142
- DOI: 10.1186/s13075-016-0921-5
Randomized controlled studies on the efficacy of antiarthritic agents in inhibiting cartilage degeneration and pain associated with progression of osteoarthritis in the rat
Abstract
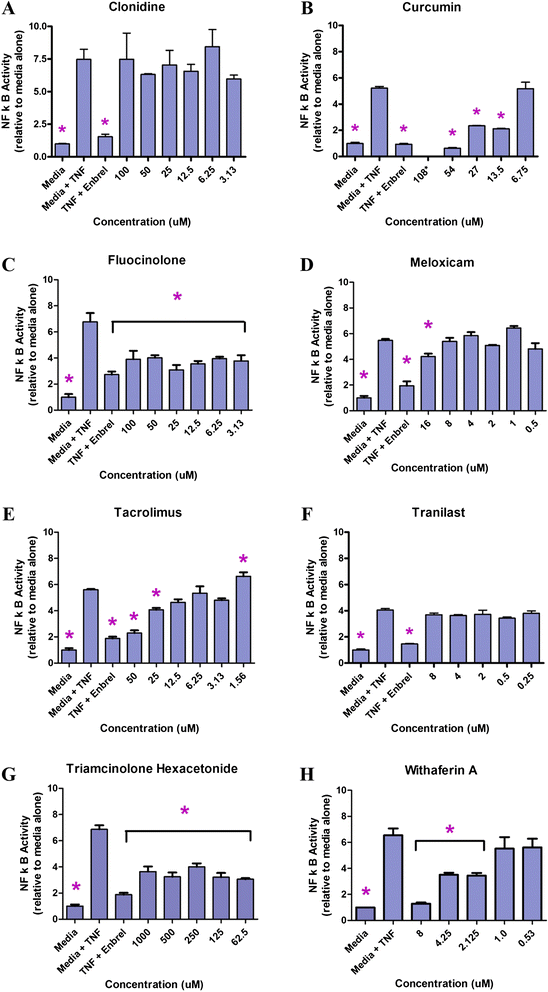
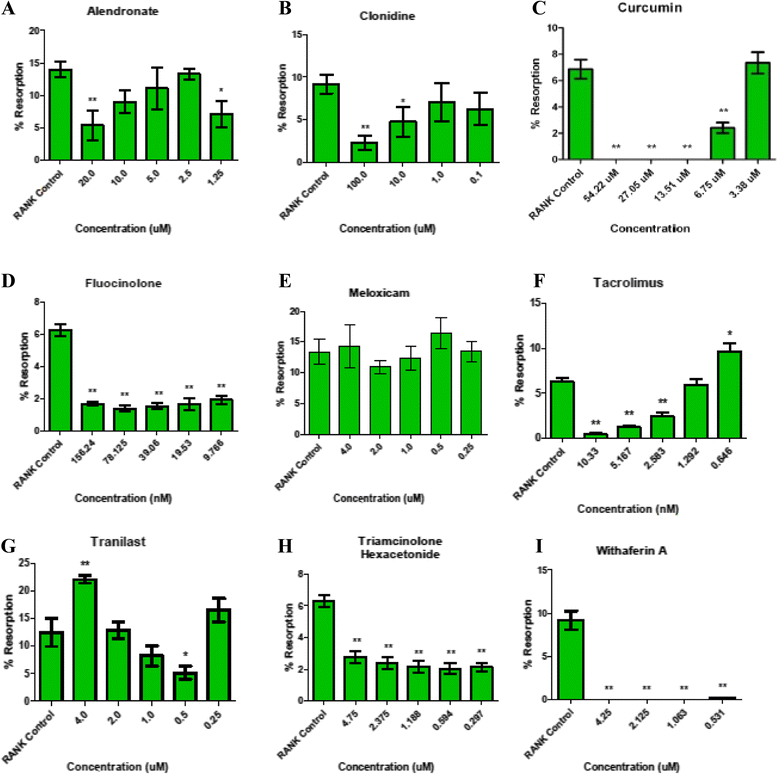
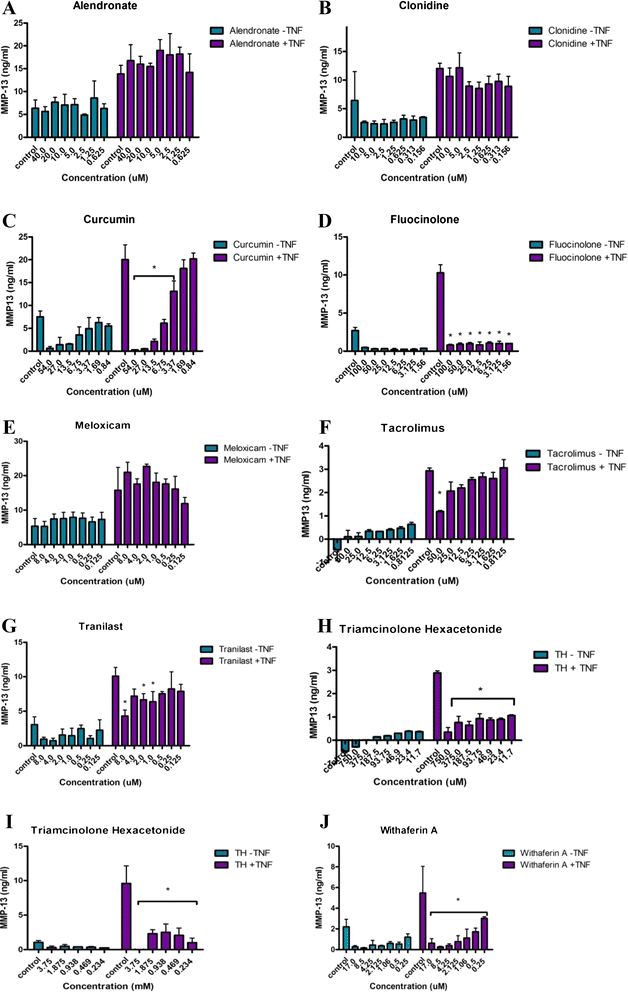
Background: As an initial step in the development of a local therapeutic to treat osteoarthritis (OA), a number of agents were tested for their ability to block activation of inflammation through nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB), subchondral bone changes through receptor activator of nuclear factor κB ligand (RANKL)-mediated osteoclastogenesis, and proteolytic degradation through matrix metalloproteinase (MMP)-13 activity. Candidates with low toxicity and predicted efficacy were further examined using either of two widely accepted models of OA joint degeneration in the rat: the monoiodoacetic acid (MIA) model or the medial meniscal tear/medial collateral ligament tear (MMT/MCLT) model.
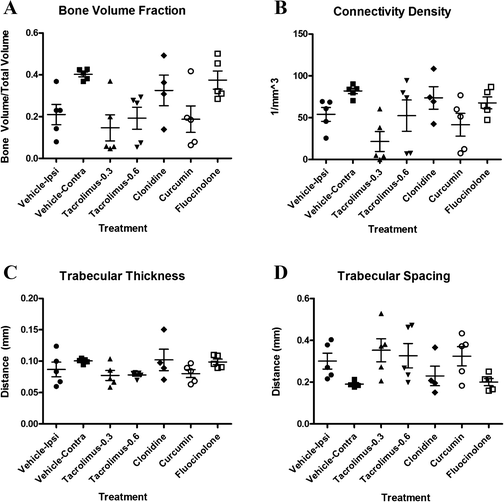
Methods: Potential therapeutics were assessed for their effects on the activation of nuclear factor (NF)-κB, RANKL-mediated osteoclastogenesis, and MMP-13 activity in vitro using previously established assays. Toxicity was measured using HeLa cells, a synovial cell line, or primary human chondrocytes. Drugs predicted to perform well in vivo were tested either systemically or via intraarticular injection in the MIA or the MMT/MCLT model of OA. Pain behavior was measured by mechanical hyperalgesia using the digital Randall-Selitto test (dRS) or by incapacitance with weight bearing (WB). Joint degeneration was evaluated using micro computed tomography and a comprehensive semiquantitative scoring of cartilage, subchondral bone, and synovial histopathology.
Results: Several agents were effective both in vitro and in vivo. With regard to pain behavior, systemically delivered clonidine was superior in treating MIA-induced changes in WB or dRS, while systemic clonidine, curcumin, tacrolimus, and fluocinolone were all somewhat effective in modifying MMT/MCLT-induced changes in WB. Systemic tacrolimus was the most effective in slowing disease progression as measured by histopathology in the MMT/MCLT model.
Conclusions: All of the agents that demonstrated highest benefit in vivo, excepting clonidine, were found to inhibit MMP-13, NF-κB, and bone matrix remodeling in vitro. The MIA and MMT/MCLT models of OA, previously shown to possess inflammatory characteristics and to display associated pain behavior, were affected to different degrees by the same drugs. Although no therapeutic was remarkable across all measures, the several which showed the most promise in either model merit continued study with alternative dosing and therapeutic strategies.
Figures

References
-
- Kotz R, Kolarz G. Intra-articular hyaluronic acid: duration of effect and results of repeated treatment cycles. Am J Orthop. 1999;28:5–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

