Challenges in circulating tumor cell detection by the CellSearch system
- PMID: 26795350
- PMCID: PMC5528971
- DOI: 10.1016/j.molonc.2015.12.002
Challenges in circulating tumor cell detection by the CellSearch system
Abstract
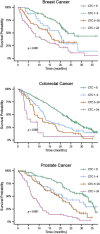
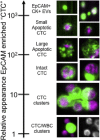
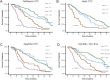
Enumeration and characterization of circulating tumor cells (CTC) hold the promise of a real time liquid biopsy. They are however present in a large background of hematopoietic cells making their isolation technically challenging. In 2004, the CellSearch system was introduced as the first and only FDA cleared method designed for the enumeration of circulating tumor cells in 7.5 mL of blood. Presence of CTC detected by CellSearch is associated with poor prognosis in metastatic carcinomas. CTC remaining in patients after the first cycles of therapy indicates a futile therapy. Here we review challenges faced during the development of the CellSearch system and the difficulties in assigning objects as CTC. The large heterogeneity of CTC and the different approaches introduced in recent years to isolate, enumerate and characterize CTC results in a large variation of the number of CTC reported urging the need for uniform definitions and at least a clear definition of what the criteria are for assigning an object as a CTC.
Keywords: CellSearch; Circulating tumor cells; Epithelial cell adhesion molecule; Rare event detection.
Copyright © 2015 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Aceto, N. , Bardia, A. , Miyamoto, D.T. , Donaldson, M.C. , Wittner, B.S. , Spencer, J.A. , Yu, M. , Pely, A. , Engstrom, A. , Zhu, H. , Brannigan, B.W. , Kapur, R. , Stott, S.L. , Shioda, T. , Ramaswamy, S. , Ting, D.T. , Lin, C.P. , Toner, M. , Haber, D.A. , Maheswaran, S. , 2014. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 158, 1110–1122. 10.1016/j.cell.2014.07.013 - DOI - PMC - PubMed
-
- Adebayo Awe, J. , Xu, M.C. , Wechsler, J. , Benali-Furet, N. , Cayre, Y.E. , Saranchuk, J. , Drachenberg, D. , Mai, S. , 2013. Three-dimensional telomeric analysis of isolated circulating tumor cells (CTCs) defines CTC subpopulations. Transl. Oncol. 6, 10.1593/tlo.12361 51–IN4 - DOI - PMC - PubMed
-
- Alix-Panabières, C. , Pantel, K. , 2014. Challenges in circulating tumour cell research. Nat. Rev. Cancer. 14, 623–631. - PubMed
-
- Allard, W.J. , Matera, J. , Miller, M.C. , Repollet, M. , Connelly, M.C. , Rao, C. , Tibbe, A.G.J. , Uhr, J.W. , Terstappen, L.W.M.M. , 2004. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 10, 6897–6904. 10.1158/1078-0432.CCR-04-0378 - DOI - PubMed
-
- Allard, W.J. , Terstappen, L.W.M.M. , 2015. CCR 20th anniversary commentary: paving the way for circulating tumor cells. Clin. Cancer Res. 21, 2883–2885. 10.1158/1078-0432.CCR-14-2559 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

