Prostaglandin E2 production during neonatal respiratory infection with mouse adenovirus type 1
- PMID: 26795547
- PMCID: PMC4764405
- DOI: 10.1016/j.virusres.2016.01.007
Prostaglandin E2 production during neonatal respiratory infection with mouse adenovirus type 1
Abstract
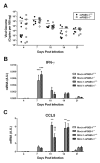
Neonatal mice are more susceptible than adults to mouse adenovirus type 1 (MAV1) respiratory infection. In adult mice, MAV-1 respiratory infection induces production of prostaglandin E2 (PGE2), a lipid mediator that exerts suppressive effects on a variety of host immune functions. We tested the hypothesis that exaggerated PGE2 production in neonatal mice contributes to increased susceptibility to MAV-1. PGE2 concentrations were lower in lungs of uninfected neonatal mice than in adults. PGE2 production was induced by both MAV-1 and a nonspecific stimulus to a greater degree in neonatal mice than in adults, but only in adults was PGE2 induced in a virus-specific manner. Lung viral loads were equivalent in PGE2-deficient neonatal mice and wild type controls, as was virus-induced expression of IFN-γ, IL-17A, and CCL5 in the lungs. PGE2 deficiency had minimal effect on production of virus-specific IgG or establishment of protective immunity in neonatal mice. Collectively, our data indicate that lung PGE2 production is exaggerated early in life, but this effect does not mediate increased susceptibility to MAV-1 infection.
Keywords: Adenovirus; Neonatal infection; Prostaglandin E(2); Viral pathogenesis.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures



References
-
- Adkins B, Bu Y, Guevara P. Murine neonatal CD4+ lymph node cells are highly deficient in the development of antigen-specific Th1 function in adoptive adult hosts. Journal of immunology. 2002;169(9):4998–5004. - PubMed
-
- Adkins B, Du RQ. Newborn mice develop balanced Th1/Th2 primary effector responses in vivo but are biased to Th2 secondary responses. Journal of immunology. 1998;160(9):4217–4224. - PubMed
-
- Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. Journal of immunology. 2004;173(1):559–565. - PubMed
-
- Aronoff DM, Canetti C, Serezani CH, Luo M, Peters-Golden M. Cutting edge: macrophage inhibition by cyclic AMP (cAMP): differential roles of protein kinase A and exchange protein directly activated by cAMP-1. Journal of immunology. 2005;174(2):595–599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

