Development and Validation of a Clostridium difficile Health-related Quality-of-Life Questionnaire
- PMID: 26796081
- PMCID: PMC4978607
- DOI: 10.1097/MCG.0000000000000473
Development and Validation of a Clostridium difficile Health-related Quality-of-Life Questionnaire
Abstract
Goals and background: Patients with Clostridium difficile infection (CDI) can experience long-term symptoms and poor quality of life due to the disease. Despite this, a health-related quality of life (HRQOL) instrument specific for patients with CDI does not exist. The aim of this study was to develop and validate a disease-specific instrument to assess HRQOL in patients with CDI.
Study: A systematic literature review was conducted to identify HRQOL instruments and questions related to general health (n=3) or gastrointestinal disease (n=12) potentially related to CDI HRQOL. Removing duplicate questions and using direct patient or clinician interviews, a 36-item survey was developed. The survey was then tested using 98 patients with CDI and compared with the RAND Short-Form 36 (SF-36) Health Survey. Psychometric analysis techniques were used to identify domains and remove redundant items.
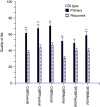
Results: Exploratory factor analysis identified 3 major domains (physical, mental, and social) with 4 associated subdomains. Survey overall and domain scores displayed good internal consistency (Cronbach α coefficient >0.87) and concurrent validity evidenced by significant correlation with SF-36 scores. The C. difficile survey scores were better able than the SF-36 to discriminate quality-of-life score differences in patients with primary versus recurrent CDI and increasing time since last episode of CDI. The final version contained 32 items related to the physical, mental, and social health of CDI patients.
Conclusion: The properties of the newly developed Cdiff32 should make it appropriate to assess changes over time in HRQOL in patients with CDI.
Conflict of interest statement
D.N.S. has ongoing research support from Merck and Cubist; H.L.D. reports receiving funding from Actelion; K.W.G. reports receiving funding from Cubist, Summit, and Merck. The remaining authors declare that they have nothing to disclose.
Figures
References
-
- Miller BA, Chen LF, Sexton DJ, et al. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32:387–390. - PubMed
-
- Frieden T. Antibiotic resistance threats in the United States. 2013. Available at: http://www.cdc.gov/drugresistance/threat-report-2013/. editor. CDC.gov2013. Accessed January 6, 2016.
-
- Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med. 2008;359:1932–1940. - PubMed
-
- Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18(suppl 6):21–27. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources