Inner-membrane protein MorC is involved in fimbriae production and biofilm formation in Aggregatibacter actinomycetemcomitans
- PMID: 26796329
- PMCID: PMC4891992
- DOI: 10.1099/mic.0.000246
Inner-membrane protein MorC is involved in fimbriae production and biofilm formation in Aggregatibacter actinomycetemcomitans
Abstract
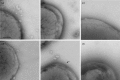
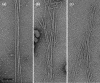
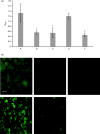
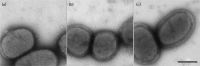
Fimbrial subunit synthesis, secretion and assembly on the surface of the periodontal pathogen Aggregatibacter actinomycetemcomitans are essential for biofilm formation. A recent quantitative proteomics study employing an afimbriated strain and a developed mutant isogenic for the inner-membrane protein morphogenesis protein C (MorC) revealed that the abundance of the proteins of the fimbrial secretion apparatus in the membrane is dependent on MorC. To investigate further the relationship between MorC and fimbriation, we identified and complemented the defect in fimbriae production in the afimbriated laboratory strain. The transformed strain expressing a plasmid containing genes encoding the WT fimbrial subunit and the prepilin peptidase displayed all of the hallmarks of a fimbriated bacterium including the distinct star-like colony morphology, robust biofilm formation, biofilm architecture composed of discrete microcolonies and the presence of fimbriae. When the identical plasmid was transformed into a morC mutant strain, the bacterium did not display any of the phenotypes of fimbriated strains. Extension of these studies to a naturally fimbriated clinical strain showed that the resulting morC mutant maintained the characteristic colony morphology of fimbriated strains. There was, however, a reduction in the secretion of fimbrial subunits, and fewer fimbriae were observed on the surface of the mutant strain. Furthermore, the morC mutant of the fimbriated strain displayed a significantly altered biofilm microcolony architecture, while maintaining a similar biofilm mass to the parent strain. These results suggest that MorC influences fimbrial secretion and microcolony formation in A. actinomycetemcomitans.
Figures



Similar articles
-
The conserved carboxyl domain of MorC, an inner membrane protein of Aggregatibacter actinomycetemcomitans, is essential for membrane function.Mol Oral Microbiol. 2016 Feb;31(1):43-58. doi: 10.1111/omi.12120. Epub 2015 Sep 15. Mol Oral Microbiol. 2016. PMID: 26205976 Free PMC article.
-
A Nonfimbrial Adhesin of Aggregatibacter actinomycetemcomitans Mediates Biofilm Biogenesis.Infect Immun. 2018 Dec 19;87(1):e00704-18. doi: 10.1128/IAI.00704-18. Print 2019 Jan. Infect Immun. 2018. PMID: 30297525 Free PMC article.
-
Alteration in abundance of specific membrane proteins of Aggregatibacter actinomycetemcomitans is attributed to deletion of the inner membrane protein MorC.Proteomics. 2015 Jun;15(11):1859-67. doi: 10.1002/pmic.201400505. Epub 2015 Mar 17. Proteomics. 2015. PMID: 25684173 Free PMC article.
-
Genes for tight adherence of Actinobacillus actinomycetemcomitans: from plaque to plague to pond scum.Trends Microbiol. 2001 Sep;9(9):429-37. doi: 10.1016/s0966-842x(01)02161-8. Trends Microbiol. 2001. PMID: 11553455 Review.
-
MORC protein family-related signature within human disease and cancer.Cell Death Dis. 2021 Nov 27;12(12):1112. doi: 10.1038/s41419-021-04393-1. Cell Death Dis. 2021. PMID: 34839357 Free PMC article. Review.
Cited by
-
Identification and Characterization of an Aeromonas hydrophila Oligopeptidase Gene pepF Negatively Related to Biofilm Formation.Front Microbiol. 2016 Sep 22;7:1497. doi: 10.3389/fmicb.2016.01497. eCollection 2016. Front Microbiol. 2016. PMID: 27713736 Free PMC article.
-
The Trimeric Autotransporter Adhesin EmaA and Infective Endocarditis.Pathogens. 2024 Jan 23;13(2):99. doi: 10.3390/pathogens13020099. Pathogens. 2024. PMID: 38392837 Free PMC article. Review.
-
Investigating the antimicrobial and anti-inflammatory effects of Lactobacillus and Bifidobacterium spp. on cariogenic and periodontitis pathogens.Front Microbiol. 2024 May 30;15:1383959. doi: 10.3389/fmicb.2024.1383959. eCollection 2024. Front Microbiol. 2024. PMID: 38881669 Free PMC article.
-
A conserved C-terminal domain of TamB interacts with multiple BamA POTRA domains in Borreliella burgdorferi.PLoS One. 2024 Aug 29;19(8):e0304839. doi: 10.1371/journal.pone.0304839. eCollection 2024. PLoS One. 2024. PMID: 39208212 Free PMC article.
-
Effect of Antimicrobial Photodynamic Therapy Using Indocyanine Green Doped with Chitosan Nanoparticles on Biofilm Formation-Related Gene Expression of Aggregatibacter actinomycetemcomitans.Front Dent. 2019 May-Jun;16(3):187-193. doi: 10.18502/fid.v16i3.1590. Epub 2019 Jun 29. Front Dent. 2019. PMID: 31858084 Free PMC article.
References
-
- Bodet C., Andrian E., Tanabe S., Grenier D. (2007). Actinobacillus actinomycetemcomitans lipopolysaccharide regulates matrix metalloproteinase, tissue inhibitors of matrix metalloproteinase, and plasminogen activator production by human gingival fibroblasts: a potential role in connective tissue destruction J Cell Physiol 212 189–194 10.1002/jcp.21018 . - DOI - PubMed
-
- Burall L. S., Harro J. M., Li X., Lockatell C. V., Himpsl S. D., Hebel J. R., Johnson D. E., Mobley H. L. (2004). Proteus mirabilis genes that contribute to pathogenesis of urinary tract infection: identification of 25 signature-tagged mutants attenuated at least 100-fold Infect Immun 72 2922–2938 10.1128/IAI.72.5.2922-2938.2004 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

