Membrane omega-3 fatty acids modulate the oligomerisation kinetics of adenosine A2A and dopamine D2 receptors
- PMID: 26796668
- PMCID: PMC4726318
- DOI: 10.1038/srep19839
Membrane omega-3 fatty acids modulate the oligomerisation kinetics of adenosine A2A and dopamine D2 receptors
Abstract
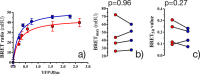
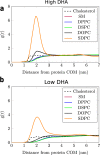
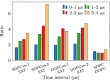
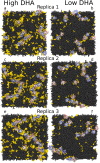
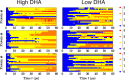
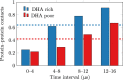
Membrane levels of docosahexaenoic acid (DHA), an essential omega-3 polyunsaturated fatty acid (ω-3 PUFA), are decreased in common neuropsychiatric disorders. DHA modulates key cell membrane properties like fluidity, thereby affecting the behaviour of transmembrane proteins like G protein-coupled receptors (GPCRs). These receptors, which have special relevance for major neuropsychiatric disorders have recently been shown to form dimers or higher order oligomers, and evidence suggests that DHA levels affect GPCR function by modulating oligomerisation. In this study, we assessed the effect of membrane DHA content on the formation of a class of protein complexes with particular relevance for brain disease: adenosine A2A and dopamine D2 receptor oligomers. Using extensive multiscale computer modelling, we find a marked propensity of DHA for interaction with both A2A and D2 receptors, which leads to an increased rate of receptor oligomerisation. Bioluminescence resonance energy transfer (BRET) experiments performed on living cells suggest that this DHA effect on the oligomerisation of A2A and D2 receptors is purely kinetic. This work reveals for the first time that membrane ω-3 PUFAs play a key role in GPCR oligomerisation kinetics, which may have important implications for neuropsychiatric conditions like schizophrenia or Parkinson's disease.
Figures

References
-
- Martín V. et al. Lipid alterations in lipid rafts from Alzheimer’s disease human brain cortex. J. Alzheimers Dis. 19, 489–502 (2010). - PubMed
-
- Innis S. M. Dietary omega 3 fatty acids and the developing brain. Brain Res. 1237, 35–43 (2008). - PubMed
-
- Calon F. & Cole G. Neuroprotective action of omega-3 polyunsaturated fatty acids against neurodegenerative diseases: evidence from animal studies. Prostag. Leukotr. Ess. 77, 287–293 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

