Modeling Staphylococcus epidermidis-Induced Non-Unions: Subclinical and Clinical Evidence in Rats
- PMID: 26796958
- PMCID: PMC4721651
- DOI: 10.1371/journal.pone.0147447
Modeling Staphylococcus epidermidis-Induced Non-Unions: Subclinical and Clinical Evidence in Rats
Abstract
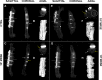
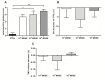
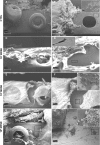
S. epidermidis is one of the leading causes of orthopaedic infections associated with biofilm formation on implant devices. Open fractures are at risk of S. epidermidis transcutaneous contamination leading to higher non-union development compared to closed fractures. Although the role of infection in delaying fracture healing is well recognized, no in vivo models investigated the impact of subclinical low-grade infections on bone repair and non-union. We hypothesized that the non-union rate is directly related to the load of this commonly retrieved pathogen and that a low-grade contamination delays the fracture healing without clinically detectable infection. Rat femurs were osteotomized and stabilized with plates. Fractures were infected with a characterized clinical-derived methicillin-resistant S. epidermidis (10(3), 10(5), 10(8) colony forming units) and compared to uninfected controls. After 56 days, bone healing and osteomyelitis were clinically assessed and further evaluated by micro-CT, microbiological and histological analyses. The biofilm formation was visualized by scanning electron microscopy. The control group showed no signs of infection and a complete bone healing. The 10(3) group displayed variable response to infection with a 67% of altered bone healing and positive bacterial cultures, despite no clinical signs of infection present. The 10(5) and 10(8) groups showed severe signs of osteomyelitis and a non-union rate of 83-100%, respectively. The cortical bone reaction related to the periosteal elevation in the control group and the metal scattering detected by micro-CT represented limitations of this study. Our model showed that an intra-operative low-grade S. epidermidis contamination might prevent the bone healing, even in the absence of infectious signs. Our findings also pointed out a dose-dependent effect between the S. epidermidis inoculum and non-union rate. This pilot study identifies a relevant preclinical model to assess the role of subclinical infections in orthopaedic and trauma surgery and to test specifically designed diagnostic, prevention and therapeutic strategies.
Conflict of interest statement
Figures




References
-
- Alt V, Lips KS, Henkenbehrens C, Muhrer D, Oliveira Cavalcanti MC, Sommer U, et al. A new animal model for implant-related infected non-unions after intramedullary fixation of the tibia in rats with fluorescent in situ hybridization of bacteria in bone infection. Bone 2011;48:1146–1153. 10.1016/j.bone.2011.01.018 - DOI - PubMed
-
- Trampuz A, Zimmerli W. Diagnosis and treatment of implant associated septic arthritis and osteomyelitis. Curr Infect Dis Rep 2008;10:394–403. - PubMed
-
- Ostermann PA, Henry SL, Seligson D. Timing of wound closure in severe compound fractures. Orthopedics 1994;17:397–399. - PubMed
-
- Seligson D, Klemm K. Adult posttraumatic osteomyelitis of the tibial diaphysis of the tibial shaft. Clin Orthop Relat Res 1999;360:30–36. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

