Oxidative Stress Promotes Peroxiredoxin Hyperoxidation and Attenuates Pro-survival Signaling in Aging Chondrocytes
- PMID: 26797130
- PMCID: PMC4807251
- DOI: 10.1074/jbc.M115.693523
Oxidative Stress Promotes Peroxiredoxin Hyperoxidation and Attenuates Pro-survival Signaling in Aging Chondrocytes
Abstract
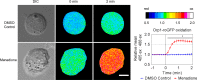
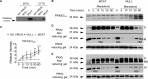
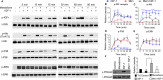
Oxidative stress-mediated post-translational modifications of redox-sensitive proteins are postulated as a key mechanism underlying age-related cellular dysfunction and disease progression. Peroxiredoxins (PRX) are critical intracellular antioxidants that also regulate redox signaling events. Age-related osteoarthritis is a common form of arthritis that has been associated with mitochondrial dysfunction and oxidative stress. The objective of this study was to determine the effect of aging and oxidative stress on chondrocyte intracellular signaling, with a specific focus on oxidation of cytosolic PRX2 and mitochondrial PRX3. Menadione was used as a model to induce cellular oxidative stress. Compared with chondrocytes isolated from young adult humans, chondrocytes from older adults exhibited higher levels of PRX1-3 hyperoxidation basally and under conditions of oxidative stress. Peroxiredoxin hyperoxidation was associated with inhibition of pro-survival Akt signaling and stimulation of pro-death p38 signaling. These changes were prevented in cultured human chondrocytes by adenoviral expression of catalase targeted to the mitochondria (MCAT) and in cartilage explants from MCAT transgenic mice. Peroxiredoxin hyperoxidation was observedin situin human cartilage sections from older adults and in osteoarthritic cartilage. MCAT transgenic mice exhibited less age-related osteoarthritis. These findings demonstrate that age-related oxidative stress can disrupt normal physiological signaling and contribute to osteoarthritis and suggest peroxiredoxin hyperoxidation as a potential mechanism.
Keywords: aging; cell signaling; osteoarthritis; oxidative stress; peroxiredoxin; redox signaling.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures






Similar articles
-
Differential peroxiredoxin hyperoxidation regulates MAP kinase signaling in human articular chondrocytes.Free Radic Biol Med. 2019 Apr;134:139-152. doi: 10.1016/j.freeradbiomed.2019.01.005. Epub 2019 Jan 9. Free Radic Biol Med. 2019. PMID: 30639614 Free PMC article.
-
Articular chondrocytes isolated from the knee and ankle joints of human tissue donors demonstrate similar redox-regulated MAP kinase and Akt signaling.Osteoarthritis Cartilage. 2019 Apr;27(4):703-711. doi: 10.1016/j.joca.2018.12.010. Epub 2018 Dec 24. Osteoarthritis Cartilage. 2019. PMID: 30590195 Free PMC article.
-
Mitochondrial pathology in osteoarthritic chondrocytes.Curr Drug Targets. 2014;15(7):710-9. doi: 10.2174/1389450115666140417120305. Curr Drug Targets. 2014. PMID: 24745822 Review.
-
Reactive oxygen species, aging and articular cartilage homeostasis.Free Radic Biol Med. 2019 Feb 20;132:73-82. doi: 10.1016/j.freeradbiomed.2018.08.038. Epub 2018 Aug 31. Free Radic Biol Med. 2019. PMID: 30176344 Free PMC article. Review.
-
ROS/oxidative stress signaling in osteoarthritis.Biochim Biophys Acta. 2016 Apr;1862(4):576-591. doi: 10.1016/j.bbadis.2016.01.003. Epub 2016 Jan 6. Biochim Biophys Acta. 2016. PMID: 26769361 Review.
Cited by
-
Age and oxidative stress regulate Nrf2 homeostasis in human articular chondrocytes.Osteoarthritis Cartilage. 2023 Sep;31(9):1214-1223. doi: 10.1016/j.joca.2023.05.004. Epub 2023 May 7. Osteoarthritis Cartilage. 2023. PMID: 37160250 Free PMC article.
-
Oxymatrine protects articular chondrocytes from IL-1β-induced damage through autophagy activation via AKT/mTOR signaling pathway inhibition.J Orthop Surg Res. 2024 Mar 11;19(1):178. doi: 10.1186/s13018-024-04667-2. J Orthop Surg Res. 2024. PMID: 38468339 Free PMC article.
-
Ginkgolide C slows the progression of osteoarthritis by activating Nrf2/HO-1 and blocking the NF-κB pathway.Front Pharmacol. 2022 Oct 28;13:1027553. doi: 10.3389/fphar.2022.1027553. eCollection 2022. Front Pharmacol. 2022. PMID: 36386227 Free PMC article.
-
Beneficial Effect of Tempol, a Membrane-Permeable Radical Scavenger, on Inflammation and Osteoarthritis in In Vitro Models.Biomolecules. 2021 Feb 25;11(3):352. doi: 10.3390/biom11030352. Biomolecules. 2021. PMID: 33669093 Free PMC article.
-
Enzymatic Depletion of Mitochondrial Inorganic Polyphosphate (polyP) Increases the Generation of Reactive Oxygen Species (ROS) and the Activity of the Pentose Phosphate Pathway (PPP) in Mammalian Cells.Antioxidants (Basel). 2022 Mar 31;11(4):685. doi: 10.3390/antiox11040685. Antioxidants (Basel). 2022. PMID: 35453370 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

