Efavirenz but Not Atazanavir/Ritonavir Significantly Reduces Atovaquone Concentrations in HIV-Infected Subjects
- PMID: 26797214
- PMCID: PMC4803107
- DOI: 10.1093/cid/ciw028
Efavirenz but Not Atazanavir/Ritonavir Significantly Reduces Atovaquone Concentrations in HIV-Infected Subjects
Abstract
Background: The current study was conducted to determine if efavirenz (EFV) or atazanavir/ritonavir (ATV/r)-based combination antiretroviral therapy (cART) impacted steady-state atovaquone plasma concentrations in human immunodeficiency virus (HIV)-infected patients receiving treatment doses of atovaquone.
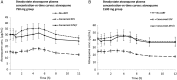
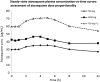
Methods: Thirty HIV-infected volunteers were recruited, 10 taking no cART and 10 each taking cART that included EFV or ATV/r. Subjects were randomly assigned to atovaquone 750 mg twice daily (BID) for 14 days followed by atovaquone 1500 mg BID for 14 days, or vice-versa, with a washout period in between. On day 14 of each phase, blood was sampled for pharmacokinetic studies, and the area under the concentration-time curve (AUCτ) and average concentration (C avg) were calculated and compared using an unpaired t test.
Results: Twenty-nine subjects completed both dosing cohorts. Subjects receiving EFV-based cART had 47% and 44% lower atovaquone AUCτ than subjects not receiving cART at atovaquone doses of 750 mg BID and 1500 mg BID, respectively (P≤ .01). Only 5 of 10 subjects receiving EFV-based cART plus atovaquone 750 mg BID had an atovaquone C avg>15 µg/mL, which has previously been associated with successful treatment of Pneumocystis jirovecipneumonia. AUCτ and Cavg did not significantly differ for concurrent ATV/r for 750 mg BID or 1500 mg BID when compared to the group not receiving cART. Nine of 10 subjects not receiving cART, 8 of 10 subjects receiving ATV/r, and 2 of 10 subjects receiving EFV in combination with atovaquone 750 mg BID achieved an atovaquone C avg>18.5 µg/mL, a concentration that has previously been associated with successful treatment of Toxoplasmaencephalitis (TE).
Conclusions: These data suggest that the currently recommended dose of atovaquone 750 mg BID for treatment of mild to moderate PCP may not be adequate in patients receiving concurrent EFV. Furthermore, doses lower than the currently recommended dose of 1500 mg BID may achieve plasma concentrations adequate to treat TE in HIV-infected patients not receiving EFV.
Clinical trials registration: NCT01479361.
Keywords: Pneumocystis jiroveci pneumonia; atovaquone; drug interaction; efavirenz; toxoplasma encephalitis.
Published by Oxford University Press for the Infectious Diseases Society of America 2016. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures

References
-
- Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf Accessed 1 February 2016. - PubMed
-
- GlaxoSmithKline. Mepron [package insert]. Research Triangle Park, NC: GlaxoSmithKline, 2008.
-
- Torres RA, Weinberg W, Stansell J et al. . Atovaquone for salvage treatment and suppression of toxoplasmic encephalitis in patients with AIDS. Clin Infect Dis 1997; 24:422–9. - PubMed
-
- Hughes W, Leoung G, Kramer F et al. . Comparison of atovaquone (566C80) with trimethoprim-sulfamethoxazole to treat Pneumocystis carinii pneumonia in patients with AIDS. N Engl J Med 1993; 328:1521–7. - PubMed
-
- van der Lee MJ, Dawood L, ter Hofstede HJ et al. . Lopinavir/ritonavir reduces lamotrigine plasma concentrations in healthy subjects. Clin Pharmacol Ther 2006; 80:159–68. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

