Genipin Derivatives Protect RGC-5 from Sodium Nitroprusside-Induced Nitrosative Stress
- PMID: 26797604
- PMCID: PMC4730358
- DOI: 10.3390/ijms17010117
Genipin Derivatives Protect RGC-5 from Sodium Nitroprusside-Induced Nitrosative Stress
Abstract
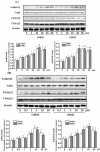
CHR20 and CHR21 are a pair of stable diastereoisomers derived from genipin. These stereoisomers are activators of neuronal nitric oxide synthase (nNOS) and endothelial nitric oxide synthase (eNOS). In the rat retinal ganglion (RGC-5) cell model these compounds are non-toxic. Treatment of RGC-5 with 750 μM of sodium nitroprusside (SNP) produces nitrosative stress. Both genipin derivatives, however, protect these cells against SNP-induced apoptic cell death, although CHR21 is significantly more potent than CHR20 in this regard. With Western blotting we showed that the observed neuroprotection is primarily due to the activation of protein kinase B (Akt)/eNOS and extracellular signal-regulated kinase (ERK1/2) signaling pathways. Therefore, LY294002 (a phosphatidylinositol 3-kinase (PI3K) inhibitor) or PD98059 (a MAPK-activating enzyme inhibitor) abrogated the protective effects of CHR20 and CHR21. Altogether, our results show that in our experimental setup neuroprotection by the diasteromeric pair is mediated through the PI3K/Akt/eNOS and ERK1/2 signaling pathways. Further studies are needed to establish the potential of these compounds to prevent ntric oxide (NO)-induced toxicity commonly seen in many neurodegenerative diseases.
Keywords: NO neurotoxicity; apoptosis; genipin; neurodegeneration; neuroprotection; nitric oxide synthase.
Figures









References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

