Subcutaneous rapid-acting insulin analogues for diabetic ketoacidosis
- PMID: 26798030
- PMCID: PMC8829395
- DOI: 10.1002/14651858.CD011281.pub2
Subcutaneous rapid-acting insulin analogues for diabetic ketoacidosis
Abstract
Background: Diabetic ketoacidosis (DKA) is an acute, life-threatening complication of uncontrolled diabetes that mainly occurs in individuals with autoimmune type 1 diabetes, but it is not uncommon in some people with type 2 diabetes. The treatment of DKA is traditionally accomplished by the administration of intravenous infusion of regular insulin that is initiated in the emergency department and continued in an intensive care unit or a high-dependency unit environment. It is unclear whether people with DKA should be treated with other treatment modalities such as subcutaneous rapid-acting insulin analogues.
Objectives: To assess the effects of subcutaneous rapid-acting insulin analogues for the treatment of diabetic ketoacidosis.
Search methods: We identified eligible trials by searching MEDLINE, PubMed, EMBASE, LILACS, CINAHL, and the Cochrane Library. We searched the trials registers WHO ICTRP Search Portal and ClinicalTrials.gov. The date of last search for all databases was 27 October 2015. We also examined reference lists of included randomised controlled trials (RCTs) and systematic reviews, and contacted trial authors.
Selection criteria: We included trials if they were RCTs comparing subcutaneous rapid-acting insulin analogues versus standard intravenous infusion in participants with DKA of any age or sex with type 1 or type 2 diabetes, and in pregnant women.
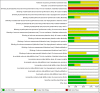
Data collection and analysis: Two review authors independently extracted data, assessed studies for risk of bias, and evaluated overall study quality utilising the GRADE instrument. We assessed the statistical heterogeneity of included studies by visually inspecting forest plots and quantifying the diversity using the I² statistic. We synthesised data using random-effects model meta-analysis or descriptive analysis, as appropriate.
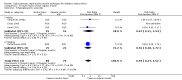
Main results: Five trials randomised 201 participants (110 participants to subcutaneous rapid-acting insulin analogues and 91 to intravenous regular insulin). The criteria for DKA were consistent with the American Diabetes Association criteria for mild or moderate DKA. The underlying cause of DKA was mostly poor compliance with diabetes therapy. Most trials did not report on type of diabetes. Younger diabetic participants and children were underrepresented in our included trials (one trial only). Four trials evaluated the effects of the rapid-acting insulin analogue lispro, and one the effects of the rapid-acting insulin analogue aspart. The mean follow-up period as measured by mean hospital stay ranged between two and seven days. Overall, risk of bias of the evaluated trials was unclear in many domains and high for performance bias for the outcome measure time to resolution of DKA.No deaths were reported in the included trials (186 participants; 3 trials; moderate- (insulin lispro) to low-quality evidence (insulin aspart)). There was very low-quality evidence to evaluate the effects of subcutaneous insulin lispro versus intravenous regular insulin on the time to resolution of DKA: mean difference (MD) 0.2 h (95% CI -1.7 to 2.1); P = 0.81; 90 participants; 2 trials. In one trial involving children with DKA, the time to reach a glucose level of 250 mg/dL was similar between insulin lispro and intravenous regular insulin. There was very low-quality evidence to evaluate the effects of subcutaneous insulin aspart versus intravenous regular insulin on the time to resolution of DKA: MD -1 h (95% CI -3.2 to 1.2); P = 0.36; 30 participants; 1 trial. There was low-quality evidence to evaluate the effects of subcutaneous rapid-acting insulin analogues versus intravenous regular insulin on hypoglycaemic episodes: 6 of 80 insulin lispro-treated participants compared with 9 of 76 regular insulin-treated participants reported hypoglycaemic events; risk ratio (RR) 0.59 (95% CI 0.23 to 1.52); P = 0.28; 156 participants; 4 trials. For insulin aspart compared with regular insulin, RR for hypoglycaemic episodes was 1.00 (95% CI 0.07 to 14.55); P = 1.0; 30 participants; 1 trial; low-quality evidence. Socioeconomic effects as measured by length of mean hospital stay for insulin lispro compared with regular insulin showed a MD of -0.4 days (95% CI -1 to 0.2); P = 0.22; 90 participants; 2 trials; low-quality evidence and for insulin aspart compared with regular insulin 1.1 days (95% CI -3.3 to 1.1); P = 0.32; low-quality evidence. Data on morbidity were limited, but no specific events were reported for the comparison of insulin lispro with regular insulin. No trial reported on adverse events other than hypoglycaemic episodes, and no trial investigated patient satisfaction.
Authors' conclusions: Our review, which provided mainly data on adults, suggests on the basis of mostly low- to very low-quality evidence that there are neither advantages nor disadvantages when comparing the effects of subcutaneous rapid-acting insulin analogues versus intravenous regular insulin for treating mild or moderate DKA.
Conflict of interest statement
CAC: none known.
LCL: none known.
NDF: none known.
DGP: none known.
Figures
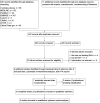








Update of
References
References to studies included in this review
Della Manna 2005 {published data only}
-
- Della Manna T, Steinmetz L, Campos PR, Farhat SC, Schvartsman C, Kuperman H, et al. Subcutaneous use of a fast‐acting insulin analog: an alternative treatment for pediatric patients with diabetic ketoacidosis. Diabetes Care 2005;28(8):1856‐61. - PubMed
Ersöz 2006 {published data only}
-
- Ersöz HO, Ukinc K, Köse M, Erem C, Gunduz A, Hacihasanoglu AB, et al. Subcutaneous lispro and intravenous regular insulin treatments are equally effective and safe for the treatment of mild and moderate diabetic ketoacidosis in adult patients. International Journal of Clinical Practice 2006;60(4):429‐33. - PubMed
Karoli 2011 {published data only}
Umpierrez 2004a {published data only}
-
- Umpierrez GE, Latif K, Stoever J, Cuervo R, Park L, Freire AX, et al. Efficacy of subcutaneous insulin lispro versus continuous intravenous regular insulin for the treatment of patients with diabetic ketoacidosis. American Journal of Medicine 2004;117(5):291‐6. - PubMed
Umpierrez 2004b {published data only}
-
- Umpierrez GE, Cuervo R, Karabell A, Latif K, Freire AX, Kitabchi AE. Treatment of diabetic ketoacidosis with subcutaneous insulin aspart. Diabetes Care 2004;27(8):1873‐8. - PubMed
References to studies excluded from this review
Adesina 2011 {published data only}
-
- Adesina OF, Kolawole BA, Ikem RT, Adebayo OJ, Soyoye DO. Comparison of lispro insulin and regular insulin in the management of hyperglycaemic emergencies. African Journal of Medicine and Medical Sciences 2011;40:59‐66. - PubMed
Armor 2011 {published data only}
Attia 1998 {published data only}
-
- Attia N, Jones TW, Holcombe J, Tamborlane WV. Comparison of human regular and lispro insulins after interruption of continuous subcutaneous insulin infusion and in the treatment of acutely decompensated IDDM. Diabetes Care 1998;5:817‐21. - PubMed
Fisher 1977 {published data only}
-
- Fisher JN, Shahshahani MN, Kitabchi AE. Diabetic ketoacidosis: low‐dose insulin therapy by various routes. The New England Journal of Medicine 1977;297(5):238‐41. - PubMed
Hsia 2011 {published data only}
-
- Hsia E, Seggelke S, Draznin B. Novel insulin infusion protocol: Subcutaneous administration of long‐acting insulin during insulin infusion prevents rebound hyperglycemia. Diabetes 2011;60:A262. - PubMed
Kadowaki 2010 {published data only}
-
- Kadowaki T, Nishida T, Kaku K. 28‐week, randomised, multicenter, open‐label, parallel‐group phase III trial to investigate the efficacy and safety of biphasic insulin aspart 70 thrice‐daily injections vs twice‐daily injections of biphasic insulin aspart 30 in patients with type 2 diabetes. Journal of Diabetes Investigation 2010;1(3):103‐10. - PMC - PubMed
Liu 2006 {published data only}
-
- Liu J, Jia Z, Zhang B, Hong LV, Zhang G, Tan B, et al. Insulin pump in the treatment of diabetic ketoacidosis. Chinese Journal of Emergency Medicine 2006;15:460‐1.
NCT00467246 {unpublished data only}
-
- NCT00467246. Sub‐cutaneous insulin in hyperglycaemic emergencies. https://www.clinicaltrials.gov/ct2/show/NCT00467246 (last accessed 4 December 2015).
NCT01365793 {unpublished data only}
-
- NCT01365793. Randomized control trial of fluid therapy for pediatric diabetic ketoacidosis. https://www.clinicaltrials.gov/ct2/show/NCT01365793 (last accessed 4 December 2015).
NCT02006342 {unpublished data only}
-
- NCT02006342. Effectiveness of subcutaneous glargine on the time to closure of the anion gap in patients presenting to the emergency department with diabetic keto‐acidosis (GT‐COG). https://www.clinicaltrials.gov/ct2/show/NCT02006342 (accessed 4 December 2015).
Philotheou 2011 {published data only}
-
- Philotheou A, Arslanian S, Blatniczky L, Peterkova V, Souhami E, Danne T. Comparable efficacy and safety of insulin glulisine and insulin lispro when given aspart of a Basal‐bolus insulin regimen in a 26‐week trial in pediatric patients with type1 diabetes. Diabetes Technology & Therapeutics 2011;3:327‐34. - PMC - PubMed
Savoldelli 2010 {published data only}
Umpierrez 2009 {published data only}
Vincent 2013 {published data only}
-
- Vincent M, Nobécourt E. Treatment of diabetic ketoacidosis with subcutaneous insulin lispro: a review of the current evidence from clinical studies. Diabetes & Metabolism 2013;39(4):299‐305. - PubMed
Weinzimer 2008 {published data only}
-
- Weinzimer SA, Ternand C, Howard C, Chang CT, Becker DJ, Laffel LM. A randomized trial comparing continuous subcutaneous insulin infusion of insulin aspart versus insulin lispro in children and adolescents with type 1 diabetes. Diabetes Care 2008;31(2):210‐5. - PubMed
References to studies awaiting assessment
El Ebrashy 2010 {published and unpublished data}
-
- Ebrashy I, Hefnawy MHMF, Basyouni A, Mahfouz H. Subcutaneous use of rapid insulin analog: an alternative treatment for patients with mild to moderate diabetic ketoacidosis. Pediatric Diabetes 2010;11(Suppl 14):32 (O/6/FRI/01).
NCT00920725 {unpublished data only}
-
- NCT00920725. Subcutaneous aspart insulin to treat diabetic ketoacidosis (DKA) and beta‐hydroxybutyrate testing in DKA. https://www.clinicaltrials.gov/ct2/show/NCT00920725 (last accessed 4 December 20015).
Additional references
ADA 1999
-
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1999;22(Suppl 1):S5‐19. - PubMed
ADA 2004
-
- American Diabetes Association. Hyperglycemic crises in diabetes. Diabetes Care 2004;27(Suppl 1):S94‐102. - PubMed
ADA 2008
-
- American Diabetes Association. Standards of medical care in diabetes ‐ 2008. Diabetes Care 2008;31(Suppl 1):S12‐54. [PUBMED: 18165335] - PubMed
Alberti 1973
-
- Alberti KG, Hockaday TD, Turner RC. Small doses of intramuscular insulin in the treatment of diabetic "coma". The Lancet 1973;2(7828):515‐22. - PubMed
Anderson 2004
-
- Anderson RT, Skovlund SE, Marrero D, Levine DW, Meadows K, Brod M, et al. Development and validation of the insulin treatment satisfaction questionnaire. Clinical Therapeutics 2004;26(4):565‐78. - PubMed
Basu 1993
-
- Basu A, Close CF, Jenkins D, Krentz AJ, Nattrass M, Wright AD. Persisting mortality in diabetic ketoacidosis. Diabetic Medicine 1993;10:282‐4. - PubMed
Beller 2013
Dave 2004
-
- Dave J, Chatterjee S, Davies M, Higgins K, Morjaria H, McNally P, et al. Evaluation of admissions and management of diabetic ketoacidosis in a large teaching hospital. Practical Diabetes International 2004;21(4):149‐53.
Faich 1983
-
- Faich GA, Fishbein HA, Ellis SE. The epidemiology of diabetic acidosis: a population‐based study. American Journal of Epidemiology 1983;117(5):551‐8. - PubMed
Hemkens 2009
Higgins 2002
-
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. - PubMed
Higgins 2003
Higgins 2009
Higgins 2011a
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
Home 2000
-
- Home PD, Lindholm A, Riis A, European Insulin Aspart Study Group. Insulin aspart vs. human insulin in the management of long‐term blood glucose control in Type 1 diabetes mellitus: a randomized controlled trial. Diabetic Medicine 2000;17(11):762‐70. - PubMed
Home 2012
-
- Home PD. The pharmacokinetics and pharmacodynamics of rapid‐acting insulin analogues and their clinical consequences. Diabetes, Obesity & Metabolism 2012;14(9):780‐8. - PubMed
Howey 1995
-
- Howey DC, Bowsher RR, Brunelle RL, Rowe HM, Santa PF, Downing‐Shelton J, et al. [Lys(B28), Pro(B29)]‐human insulin: effect of injection time on postprandial glycemia. Clinical Pharmacology and Therapeutics 1995;58(4):459‐69. - PubMed
Hróbjartsson 2013
-
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. - PMC - PubMed
Johnson 1980
-
- Johnson DD, Palumbo PJ, Chu CP. Diabetic ketoacidosis in a community‐based population. Mayo Clinic Proceedings 1980;55(2):83‐8. - PubMed
Kim 2007
-
- Kim S. Burden of hospitalizations primarily due to uncontrolled diabetes: implications of inadequate primary health care in the United States. Diabetes Care 2007;30(5):1281‐2. - PubMed
Kirkham 2010
Kitabchi 2001
-
- Kitabchi AE, Umpierrez GE, Murphy MB, Barrett EJ, Kreisberg RA, Malone JI, et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care 2001;24:131‐53. - PubMed
Kitabchi 2009
Kurtzhals 2000
-
- Kurtzhals P, Schäffer L, Sørensen A, Kristensen C, Jonassen I, Schmid C, et al. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes 2000;49(6):999‐1005. - PubMed
Liberati 2009
Luzi 1988
-
- Luzi L, Barrett EJ, Groop LC, Ferrannini E, DeFronzo RA. Metabolic effects of low‐dose insulin therapy on glucose metabolism in diabetic ketoacidosis. Diabetes 1988;37(11):1470‐7. - PubMed
Malone 1992
-
- Malone ML, Gennis V, Goodwin JS. Characteristics of diabetic ketoacidosis in older versus younger adults. Journal of the American Geriatrics Society 1992;40(11):1100‐4. - PubMed
Mathiesen 2007
-
- Mathiesen ER, Kinsley B, Amiel SA, Heller S, McCance D, Duran S, et al. Maternal glycemic control and hypoglycemia in type 1 diabetic pregnancy: a randomized trial of insulin aspart versus human insulin in 322 pregnant women. Diabetes Care 2007;30(4):771‐6. - PubMed
Mazer 2009
-
- Mazer M, Chen E. Is subcutaneous administration of rapid‐acting insulin as effective as intravenous insulin for treating diabetic ketoacidosis?. Annals of Emergency Medicine 2009;53(2):259‐63. - PubMed
Meader 2014
Muir 2004
-
- Muir AB, Quisling RG, Yang MC, Rosenbloom AL. Cerebral edema in childhood diabetic ketoacidosis: natural history, radiographic findings, and early identification. Diabetes Care 2004;27(7):1541‐6. - PubMed
Nyenwe 2011
-
- Nyenwe EA, Kitabchi AE. Evidence‐based management of hyperglycemic emergencies in diabetes mellitus. Diabetes Research and Clinical Practice 2011;94(3):340‐51. - PubMed
Rayman 2007
-
- Rayman G, Profozic V, Middle M. Insulin glulisine imparts effective glycaemic control in patients with Type 2 diabetes. Diabetes Research and Clinical Practice 2007;76(2):304‐12. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2011
-
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. - PubMed
Roach 2008
-
- Roach P. New insulin analogues and routes of delivery: pharmacodynamic and clinical considerations. Clinical Pharmacokinetics 2008;47(9):595‐610. - PubMed
Savage 2011
-
- Savage MW, Dhatariya KK, Kilvert A, Rayman G, Rees JA, Courtney CH, et al. Joint British Diabetes Societies guideline for the management of diabetic ketoacidosis. Diabetic Medicine 2011;28(5):508‐15. - PubMed
Siebenhofer 2006
Sterne 2011
-
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomized controlled trials. BMJ 2011;343:d4002. - PubMed
Warner 1998
WHO 1998
-
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15:539‐53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

