Ebola Virus Infection: Review of the Pharmacokinetic and Pharmacodynamic Properties of Drugs Considered for Testing in Human Efficacy Trials
- PMID: 26798032
- PMCID: PMC5680399
- DOI: 10.1007/s40262-015-0364-1
Ebola Virus Infection: Review of the Pharmacokinetic and Pharmacodynamic Properties of Drugs Considered for Testing in Human Efficacy Trials
Abstract
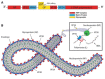
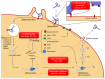
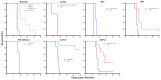
The 2014-2015 outbreak of Ebola virus disease is the largest epidemic to date in terms of the number of cases, deaths, and affected areas. In October 2015, no antiviral agents had proven antiviral efficacy in patients. However, in September 2014, the World Health Organization inventoried and has since regularly updated a list of potential drug candidates with demonstrated antiviral efficacy in in vitro or animal models. This includes agents belonging to various therapeutic classes, namely direct antiviral agents (favipiravir and BCX4430), a combination of antibodies (ZMapp), type I interferons, RNA interference-based drugs (TKM-Ebola and AVI-7537), and anticoagulant drugs (rNAPc2). Here, we review the pharmacokinetic and pharmacodynamic information presently available for these drugs, using data obtained in healthy volunteers for pharmacokinetics and data obtained in human clinical trials or animal models for pharmacodynamics. Future studies evaluating these drugs in clinical trials are critical to confirm their efficacy in humans, propose appropriate doses, and evaluate the possibility of treatment combinations.
Figures
References
-
- World Health Organization. Ebola Situation Report - 23 September 2015 | Ebola [Internet] [cited 2015 Sep 28]. Available from: http://apps.who.int/iris/bitstream/10665/185279/1/ebolasitrep_23Sept2015....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

