Long-acting muscarinic antagonists (LAMA) added to combination long-acting beta2-agonists and inhaled corticosteroids (LABA/ICS) versus LABA/ICS for adults with asthma
- PMID: 26798035
- PMCID: PMC9440477
- DOI: 10.1002/14651858.CD011721.pub2
Long-acting muscarinic antagonists (LAMA) added to combination long-acting beta2-agonists and inhaled corticosteroids (LABA/ICS) versus LABA/ICS for adults with asthma
Abstract
Background: Maintenance treatment with long-acting beta2-agonists and inhaled corticosteroids (LABA/ICS) can relieve asthma symptoms and reduce the frequency of exacerbations, but there are limited treatment options for people who do not gain control on combination LABA/ICS. Long-acting muscarinic antagonists (LAMA) are a class of inhaled drug which have been effective for people with chronic obstructive pulmonary disease and are now becoming available for people with asthma to take alongside their LABA/ICS inhaler.
Objectives: To assess the effects of adding a long-acting muscarinic antagonist (LAMA) to combination long-acting beta2-agonists (LABA) and inhaled corticosteroids (ICS) in adults whose asthma is not well controlled by LABA/ICS.
Search methods: We identified trials from the Cochrane Airways Review Group Specialised Register (CAGR) up to January 2016. We also searched ClinicalTrials.gov, the WHO trials portal, and reference lists of other reviews, and we contacted trial authors for additional information.
Selection criteria: We included parallel randomised controlled trials (RCTs) of at least 12 weeks' duration. Studies met the inclusion criteria if they compared LAMA as an add-on to LABA/ICS versus LABA/ICS alone for adults with asthma. We included studies reported as full text, those published as abstract only, and unpublished data. Primary outcomes were exacerbations requiring oral corticosteroids (OCS), validated measures of asthma control, and serious adverse events (including mortality).
Data collection and analysis: Two review authors screened searches and independently extracted details on risk of bias and numerical data. We analysed dichotomous data as odds ratios (ORs) and continuous data as mean differences (MD) using a random-effects model. We rated all outcomes using GRADE.
Main results: We found four double-blind, double-dummy trials comparing LAMA to placebo, including 1197 people with asthma taking combination LABA/ICS. One of the trials was designed to study glycopyrronium bromide but was withdrawn prior to enrolment, and the other three all studied tiotropium bromide (mostly 5 µg once daily via Respimat) over 48 to 52 weeks. People in the trials had a mean forced expiratory volume in one second (FEV1) of 55% of their predicted value, indicating severe asthma.People randomised to take tiotropium add-on had fewer exacerbations requiring oral corticosteroids than those continuing to take LABA/ICS alone, but the confidence intervals did not rule out no difference (OR 0.76, 95% CI 0.57 to 1.02; moderate quality evidence). Over 48 weeks, 328 out of 1000 people taking their usual LABA/ICS would have to take oral corticosteroids for an exacerbation compared with 271 if they took tiotropium as well (95% CI 218 to 333 per 1000). Analyses comparing the number of exacerbations per patient in each group (rate ratio) and the time until first exacerbation (hazard ratio) were in keeping with the main result. Quality of life, as measured by the Asthma Quality of Life Questionnaire (AQLQ) was no better for those taking tiotropium add-on than for those taking LABA/ICS alone when considered in light of the 0.5 minimal clinically important difference on the scale (MD 0.09, 95% CI - 0.03 to 0.20), and evidence for whether tiotropium increased or decreased serious adverse events in this population was inconsistent (OR 0.60, 95% CI 0.24 to 1.47; I(2) = 76%).Within the secondary outcomes, exacerbations requiring hospital admission were too rare to tell whether tiotropium was beneficial over LABA/ICS alone. There was high quality evidence showing benefits to lung function (trough FEV1 and forced vital capacity (FVC)) and potentially small benefits to asthma control. People taking tiotropium add-on were less likely to experience non-serious adverse events.
Authors' conclusions: Tiotropium add-on may have additional benefits over LABA/ICS alone in reducing the need for rescue oral steroids in people with severe asthma. The effect was imprecise, and there was no evidence for other LAMA preparations. Possible benefits on quality of life were negligible, and evidence for the effect on serious adverse events was inconsistent. There are likely to be small added benefits for tiotropium Respimat 5 µg daily on lung function and asthma control over LABA/ICS alone and fewer non-serious adverse events. The benefit of tiotropium add-on on the frequency of hospital admission is still unknown, despite year-long trials.Ongoing and future trials should clearly describe participants' background medications to help clinicians judge how the findings relate to stepwise care. If studies test LAMAs other than tiotropium Respimat for asthma, they should be at least six months long and use accepted and validated outcomes to allow comparisons of the safety and effectiveness between different preparations.
Conflict of interest statement
Kayleigh M. Kew: none known.
Karen Dahri: none known.
Figures
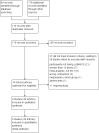
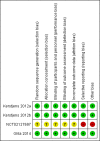












Update of
References
References to studies included in this review
Kerstjens 2012a {published and unpublished data}
-
- Beck E, Kerstjens H, Engel M, Dahl R, Paggiaro P, Vandewalker E, et al. Tiotropium Respimat add‐on therapy to inhaled corticosteroids (ICS) + long‐acting beta2‐agonists (LABAs) in patients with symptomatic severe asthma: efficacy by level of airway obstruction. Allergo Journal 2014;23(6):57.
-
- Bernstein JA, Kerstjens HAM, Moroni‐Zentgraf P, Engel M, Schmidt H, Halpin DMG. Once‐daily tiotropium is well tolerated as add‐on to standard treatment for patients with symptomatic asthma despite receiving inhaled corticosteroids and long‐acting beta2‐agonists. CHEST Annual Conference; 2013 October 26‐31; Chicago. 2013.
-
- Casale TB, Dahl R, Virchow JC, Engel M, Moroni‐Zentgraf P, Luehmann R, et al. Tiotropium respimat add‐on therapy reduces exacerbation risk in patients with symptomatic moderate to severe asthma, independent of t helper 2 status. American Thoracic Society International Conference; 2015 May 15‐20; Denver. 2015:A4193.
-
- Corren J, Frew A, Engel M, Schmidt H, Moroni‐Zentgraf P, Kerstjens HAM. Tiotropium as add‐on therapy to ICS+LABA in patients with symptomatic severe asthma: spirometric assessment over 24 hours [Abstract]. American College of Chest Physicians Annual Meeting; 2013 Oct 26‐31; Chicago. 2013; Vol. 144:91A.
-
- Corren J, Murphy KR, Bensch G, Dahl R, Paggiaro P, Engel M, et al. Once‐daily tiotropium Respimat decreases the risk of exacerbations, independent of baseline characteristics, in patients with symptomatic severe asthma without evidence of chronic obstructive pulmonary disease. Journal of General Internal Medicine 2014;29:S157‐8.
Kerstjens 2012b {published and unpublished data}
-
- 2008‐001414‐25. A Phase III randomised, double‐blind, placebo‐controlled, parallel‐group trial to evaluate efficacy and safety of tiotropium inhalation solution delivered via Respimat ® inhaler (5 μg/day) over 48 weeks as add‐on controller therapy on top of usual care in patients with severe persistent asthma. https://www.clinicaltrialsregister.eu/ctr‐search/trial/2008‐001414‐25/re... (accessed 23 June 2015).
-
- Beck E, Kerstjens H, Engel M, Dahl R, Paggiaro P, Vandewalker E, et al. Tiotropium Respimat add‐on therapy to inhaled corticosteroids (ICS) + long‐acting beta2‐agonists (LABAs) in patients with symptomatic severe asthma: efficacy by level of airway obstruction. Allergo Journal 2014;23(6):57.
-
- Bernstein JA, Kerstjens HAM, Moroni‐Zentgraf P, Engel M, Schmidt H, Halpin DMG. Once‐daily tiotropium is well tolerated as add‐on to standard treatment for patients with symptomatic asthma despite receiving inhaled corticosteroids and long‐acting beta2‐agonists. CHEST Annual Conference; 2013 October 26‐31. 2013.
-
- Casale TB, Dahl R, Virchow JC, Engel M, Moroni‐Zentgraf P, Luehmann R, et al. Tiotropium respimat add‐on therapy reduces exacerbation risk in patients with symptomatic moderate to severe asthma, independent of t helper 2 status. American Thoracic Society International Conference; 2015 May 15‐20; Denver. 2015:A4193.
-
- Corren J, Frew A, Engel M, Schmidt H, Moroni‐Zentgraf P, Kerstjens HAM. Tiotropium as add‐on therapy to ICS+LABA in patients with symptomatic severe asthma: spirometric assessment over 24 hours [Abstract]. American College of Chest Physicians Annual Meeting; 2013 Oct 26‐31; Chicago, Illinois. 2013; Vol. 144:91A.
NCT02127697 {published and unpublished data}
-
- NCT02127697. Study of efficacy and safety of NVA237 in patients with poorly controlled asthma. https://clinicaltrials.gov/ct2/show/NCT02127697 (accessed 22 June 2015).
Ohta 2014 {published and unpublished data}
-
- Ichinose M, Ohta K, Tohda Y, Engel M, Moroni‐Zentgraf P, Kunimitsu S, et al. Once‐daily tiotropium respimat is well tolerated and efficacious over 52 weeks in Japanese patients with symptomatic asthma receiving inhaled corticosteroids (ICS) + long‐acting B2‐agonist (LABA): A randomized, double‐blind, placebo‐controlled study. Respirology 2014;19(Suppl 3):65.
-
- NCT01340209. Evaluation of tiotropium 2.5 and 5 µg once daily delivered via the Respimat inhaler compared to placebo in patients with moderate to severe persistent asthma. https://clinicaltrials.gov/ct2/show/NCT01340209 (accessed 22 June 2015).
-
- Ohta K, Ichinose M, Tohda Y, Engel M, Moroni‐Zentgraf P, Kunimitsu S, et al. Long‐term once‐daily tiotropium Respimat is well tolerated and maintains efficacy over 52 weeks in patients with symptomatic asthma in Japan: A randomised, placebo‐controlled study. PLOS ONE 2015;10(4):e0124109. - PMC - PubMed
-
- Ohta K, Ichinose M, Tohda Y, Engel M, Moroni‐Zentgraf P, Kunimitsu S, et al. Once‐daily tiotropium respimat® is well tolerated and efficacious over 52 weeks in Japanese patients with symptomatic asthma receiving inhaled corticosteroids (ICS ± long‐acting Β2‐agonist (LABA): a randomized, double‐blind, placebo‐controlled study [Abstract]. American Journal of Respiratory and Critical Care Medicine 2014;189:A1311.
References to studies excluded from this review
2009‐018006‐21 {unpublished data only}
-
- 2009‐018006‐21. A phase II, randomised, double‐ blind, placebo controlled, cross‐over efficacy and safety comparison of tiotropium 5 μg administered once daily (in the evening) and tiotropium 2.5 μg administered twice daily delivered by the Respimat® inhaler for four weeks versus placebo in patients with moderate persistent asthma. https://www.clinicaltrialsregister.eu/ctr‐search/trial/2009‐018006‐21/re... (accessed 23 June 2015).
2010‐018471‐26 {unpublished data only}
-
- 2010‐018471‐26. A phase II randomised, double‐blind, placebo controlled, cross‐over efficacy and safety comparison of three doses of tiotropium inhalation solution delivered via Respimat® inhaler (1.25, 2.5 and 5.0 μg once daily) versus placebo in patients with moderate persistent asthma. https://www.clinicaltrialsregister.eu/ctr‐search/trial/2010‐018471‐26/re... (accessed 23 June 2015).
Bateman 2011 {published data only}
-
- Bateman ED, Kornmann O, Schmidt P, Pivovarova A, Engel M, Fabbri LM. Tiotropium is noninferior to salmeterol in maintaining improved lung function in B16‐Arg/Arg patients with asthma. The Journal of Allergy and Clinical Immunology 2011;128(2):315‐22. - PubMed
Beeh 2013 {published data only}
-
- Beeh KM, Ablinger O, Moroni‐Zentgraf P, Hollaenderova Z, Pivovarova A, Engel M, et al. Once‐daily add‐on tiotropium: a dose‐finding trial in adult patients with moderate persistent asthma. Allergy 2013;68(s97):376‐7.
Dusser 2014 {published data only}
-
- Dusser D, Buhl R, Castro M, Kerstjens H, Paggiaro P, Engel M, et al. Once‐daily tiotropium respimat add‐on to at least ICS in adult patients with symptomatic asthma: Pooled safety analysis. European Respiratory Journal 2014;44(Suppl 58):P905.
Fardon 2007 {published data only}
-
- Fardon T, Haggart K, Lee D K C, Lipworth B J. A proof of concept study to evaluate stepping down the dose of fluticasone in combination with salmeterol and tiotropium in severe persistent asthma. Respiratory Medicine 2007;101(6):1218‐28. - PubMed
FitzGerald 2014 {published data only}
-
- FitzGerald J M, Kerstjens H, Paggiaro P, Ohta K, Ichinose M, Moroni‐Zentgraf P, et al. Once‐daily tiotropium respimat add‐on to ICS+/‐LABA improves control across asthma severities. European Respiratory Journal 2014;44(Suppl 58):1894.
Haggart 2004 {published data only}
-
- Haggart K, Fardon TC, Lee DKC, Lipworth BJ. Stepping down inhaled steroids in severe asthma with long acting bronchodilators: utilising effort dependent and independent measures of pulmonary function [Abstract]. British Thoracic Society Winter Meeting; 2004 Dec 1‐3; London, United Kingdom. 2004; Vol. 59:ii72.
Haughney 2014 {published data only}
-
- Haughney J, Vandewalker M, Meltzer E, Paggiaro P, Engel M, Unseld A, et al. Once‐daily tiotropium Respimat: safety and tolerability results from five phase iii trials in adults with symptomatic asthma. Thorax 2014;69(Suppl 2):A178‐9.
Jiang 2006 {published data only}
-
- Jiang XP, Tan HY. Clinical observation for triple therapy in the treatment of 30 patients with bronchial asthma. Hunan Zhongyi Zazhi [Hunan Journal of Traditional Chinese Medicine] 2006;22:17‐8.
Kerstjens 2011 {published data only}
-
- Kerstjens HA, Disse B, Schröder‐Babo W, Bantje T A, Gahlemann M, Sigmund R, et al. Tiotropium improves lung function in patients with severe uncontrolled asthma: a randomized controlled trial. The Journal of Allergy and Clinical Immunology 2011;128(2):308‐14. - PubMed
Kerstjens 2015 {published data only}
-
- Casale T, Bleecker E, Meltzer E, Pizzichini E, Schmidt O, Bateman E, et al. Phase III trials to investigate tiotropium as add‐on therapy to inhaled corticosteroids for patients with symptomatic asthma: Trial design and planned statistical analyses. Allergy 2013;68(s97):377.
-
- Casale T, Carr WW, Greos L, Engel M, Bour LJ, Moroni‐Zentgraf P, et al. 24‐hour lung function response to tiotropium Respimatadd‐on to maintenance therapy in symptomatic patients with moderate persistent asthma. Annals of Allergy, Asthma & Immunology 2014;113(5):A108.
-
- Casale TB, Bateman ED, Dahl R, Pizzichini E, Vandewalker ML, Virchow JC, et al. Tiotropium respimat add‐on therapy reduces airflow obstruction in patients with symptomatic moderate asthma, independent of Th2 inflammatory status. The Journal of Allergy and Clinical Immunology 2014;133(2):AB5.
-
- Kerstjens HA, Casale TB, Bleecker ER, Meltzer EO, Pizzichini E, Schmidt O, et al. Tiotropium or salmeterol as add‐on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double‐blind, placebo‐controlled, parallel‐group, active‐comparator, randomised trials. The Lancet Respiratory Medicine 2015;3(5):367‐76. - PubMed
-
- Kerstjens HAM, Bleecker E, Meltzer E, Casale T, Pizzichini E, Schmidt O, et al. Tiotropium as add‐on to inhaled corticosteroids significantly improves asthma control as reflected by the ACQ responder rate. European Respiratory Society Annual Congress; 2013 7‐11 Sept; Barcelona. 2013.
Lee 2014 {published data only}
-
- Lee LA, Yang S, Kerwin E, Trivedi R, Edwards LD, Pascoe S. The effect of fluticasone furoate/umeclidinium in adult patients with asthma: a randomized, dose‐ranging study. Respiratory Medicine 2015;109(1):54‐62. - PubMed
Lommatzsch 2014 {published data only}
-
- Lommatzsch M, Moroni‐Zentgraf P, Cornelissen P, Unseld A, Pizzichini E, Timmer W. Tiotropium Respimat in asthma: evaluation of dosing regimens. Allergo Journal 2014;23(6):57.
Murphy 2014 {published data only}
-
- Murphy K, Bensch G, Berger WE, Engel M, Schmidt H, Moroni‐Zentgraf P, et al. Once‐daily tiotropium Respimat add‐on to inhaled corticosteroids + long‐acting beta2‐agonists improves lung function and asthma control and reduces risk of asthma worsening in patients with moderate or severe asthma. Annals of Allergy, Asthma & Immunology 2014;113(5):A107.
NCT01573624 {published data only}
-
- NCT01573624. A multi‐center, randomized, double‐blind, dose‐ranging study to evaluate GSK573719 in combination with fluticasone furoate, fluticasone furoate alone, and an active control of fluticasone furoate/vilanterol combination in subjects with asthma. http://clinicaltrials.gov/show/NCT01573624 (accessed 22 June 2015).
NCT02039011 {published data only}
-
- NCT02039011. Proof of concept study to evaluate single and chronic dosing effects of ultra‐long acting bronchodilator therapy on mannitol challenge in asthmatic patients taking inhaled corticosteroids ‐ MAN02 Ultra‐long Acting Bronchodilator Therapy in Asthmatics. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013‐001953‐28‐GB (accessed 23 June 2015).
Paggiaro 2013 {published data only}
-
- Paggiaro P, Engel M, Tudoric N, Forstner B, Radeczky E, Zubek V, et al. Phase III trial of tiotropium as add‐on therapy to low‐dose inhaled corticosteroids for patients with symptomatic mild persistent asthma: design and planned analyses [Abstract]. European Respiratory Journal 2013;42(Suppl 57):877s [P4133].
Peters 2010 {published data only}
-
- Lane M. Tiotropium bromide in asthma patients: an alternative to inhaled long‐acting beta‐agonists?. Journal of the Royal College of Physicians of Edinburgh 2010;40(4):321‐2. - PubMed
Price 2014 {published data only}
-
- Price D, Bateman ED, Paggiaro P, Kaplan A, Engel M, Schmidt H, et al. Efficacy of once‐daily tiotropium Respimat 5 mug from five phase III trials in adults with symptomatic asthma. Thorax 2014;69(Suppl 2):A50.
Rajanandh 2014 {published data only}
-
- Rajanandh MG, Nageswari A D, Ilango K. Pulmonary function assessment in mild to moderate persistent asthma patients receiving montelukast, doxofylline, and tiotropium with budesonide: a randomized controlled study. Clinical Therapeutics 2014;36(4):526‐33. - PubMed
Rajanandh 2015 {published data only}
-
- Rajanandh MG, Nageswari AD, Ilango K. Assessment of montelukast, doxofylline, and tiotropium with budesonide for the treatment of asthma: which is the best among the second‐line treatment? A randomized trial. Clinical Therapeutics 2015;37(2):418‐26. - PubMed
-
- Rajanandh MG, Nageswari AD, Ilango K. Assessment of various second‐line medications in addition to inhaled corticosteroid in asthma patients: a randomized controlled trial. Clinical and Experimental Pharmacology & Physiology 2014;41(7):509‐13. - PubMed
Rodrigo 2015 {published data only}
-
- Rodrigo GJ, Castro‐Rodriguez JA. What is the role of tiotropium in asthma?: a systematic review with meta‐analysis [Review]. Chest 2015;147(2):388‐96. - PubMed
Salvi 2009 {published data only}
-
- Salvi S, Bhosle K, Brashier B, Limaye S, Mandrekar S, Gaikwad S, et al. Eight weeks of treatment with tiotropium plus fluticasone (t + f ) produces an equally effective therapeutic response as salmeterol plus fluticasone (s + f) in subjects with moderate‐to‐severe asthma [Abstract]. European Respiratory Journal 2009;34(Suppl 53):E276.
Timmer 2014 {published data only}
-
- Timmer W, Moroni‐Zentgraf P, Cornelissen P, Unseld A, Pizzichini E, Buhl R. Once‐daily tiotropium Respimat 5 mug is an efficacious 24‐h bronchodilator in adults with symptomatic asthma. Respiratory Medicine 2015;109(3):329‐38. - PubMed
Vandewalker 2015 {published data only}
-
- Vandewalker M, Harper III T, Moroni‐Zentgraf P, Engel M, Luhmann R, Bernstein JA. Once‐daily tiotropium Respimat add‐on therapy improves lung function in adolescent patients with moderate symptomatic asthma, independent of t helper 2 inflammatory status. Annals of Allergy, Asthma & Immunology 2015;115(5 Suppl 1):A54‐5.
Vogelberg 2014 {published data only}
-
- Vogelberg C, Engel M, Moroni‐Zentgraf P, Leonaviciute‐Klimantaviciene M, Sigmund R, Downie J, et al. Once‐daily tiotropium Respimat add‐on to medium‐dose ics is an efficacious 24‐hour bronchodilator in adolescent patients with symptomatic asthma. Chest 2014;146(4):698A.
-
- Vogelberg C, Leonaviciute‐Klimantaviciene M, Vevere V, Vandewalker M, Engel M, Sigmund R, et al. Dose‐ranging study of tiotropium as treatment for moderate persistent asthma in adolescents. Allergo Journal 2013;22:389.
Yoshida 2013 {published data only}
-
- Yoshida M, Nakano T, Fukuyama S, Matsumoto T, Eguchi M, Moriwaki A, et al. Effects of tiotropium on lung function in severe asthmatics with or without emphysematous changes. Pulmonary Pharmacology and Therapeutics 2013;26(2):159‐66. - PubMed
References to ongoing studies
NCT01696214 {published data only}
-
- NCT01696214. A pilot study to determine the feasibility and utility of implementing of the full scale TOM trial (SAPS). https://clinicaltrials.gov/ct2/show/NCT01696214 (accessed 22 June 2015).
Additional references
Adams 2008a
Adams 2008b
-
- Adams NP, Bestall JC, Lasserson TJ, Jones P, Cates CJ. Fluticasone versus placebo for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003135.pub4] - DOI
Anderson 2015
BTS/SIGN 2014
-
- British Thoracic Society/Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma, October 2014. https://www.brit‐thoracic.org.uk/guidelines‐and‐quality‐standards/asthma‐guideline/ (accessed 16 March 2015).
Ducharme 2008
-
- Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ. Addition of long‐acting beta2‐agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2008, Issue 5. [DOI: 10.1002/14651858.CD005535.pub2] - DOI - PMC - PubMed
Ducharme 2010
-
- Ducharme FM, Ni Choinin M, Greenstone I, Lasserson TJ. Addition of long‐acting beta2‐agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD005533.pub2] - DOI - PMC - PubMed
eMC 2014
-
- Electronic Medicines Compendium. License extension 19th September 2014: Spiriva Respimat 2.5 micrograms solution for inhalation. https://www.medicines.org.uk/emc/history/20134 (accessed 16 March 2015).
GINA 2014
-
- Global Initiative for Asthma. Global strategy for asthma management and prevention. http://www.ginasthma.org/ (accessed 16 March 2015).
Global Asthma Report 2011
-
- International Union Against Tuberculosis and Lung Disease. The Global Asthma Report 2011. http://theunion.org/what‐we‐do/publications/technical/global‐asthma‐report (accessed 16 March 2015).
GRADEpro [Computer program]
-
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. Hamilton, Ontario: McMaster University, 2008.
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Kerstjens 2012
-
- Kerstjens HAM, Engel M, Bateman ED. Tiotropium in asthma [correspondence]. New England Journal of Medicine 2012;367:2552‐3. - PubMed
Lipworth 1999
-
- Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy. Archives of Internal Medicine 1999;159(9):941‐55. - PubMed
Lipworth 2014
Moher 2009
Moulton 2011
NRAD 2014
-
- Royal College of Physicians. Why asthma still kills: the national review of asthma deaths. www.rcplondon.ac.uk/sites/default/files/why‐asthma‐still‐kills‐full‐repo... (accessed 27 March 2015).
Pandya 2014
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Therapeutic Choices 2014
-
- McIvor RA. Respiratory disorders: chronic obstructive pulmonary disease. In: Gray J editor(s). Therapeutic Choices. 7th Edition. Ottawa: Canadian Pharmaceutical Association, 2014.
Tian 2014
-
- Tian J, Chen J, Chen R, Chen X. Tiotropium versus placebo for inadequately controlled asthma: a meta‐analysis. Respiratory Care 2014;59(5):654‐66. - PubMed
References to other published versions of this review
Kew 2015
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

