Genetic and Epigenetic Mechanisms That Maintain Hematopoietic Stem Cell Function
- PMID: 26798358
- PMCID: PMC4699043
- DOI: 10.1155/2016/5178965
Genetic and Epigenetic Mechanisms That Maintain Hematopoietic Stem Cell Function
Abstract
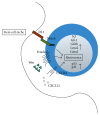
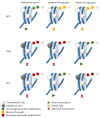
All hematopoiesis cells develop from multipotent progenitor cells. Hematopoietic stem cells (HSC) have the ability to develop into all blood lineages but also maintain their stemness. Different molecular mechanisms have been identified that are crucial for regulating quiescence and self-renewal to maintain the stem cell pool and for inducing proliferation and lineage differentiation. The stem cell niche provides the microenvironment to keep HSC in a quiescent state. Furthermore, several transcription factors and epigenetic modifiers are involved in this process. These create modifications that regulate the cell fate in a more or less reversible and dynamic way and contribute to HSC homeostasis. In addition, HSC respond in a unique way to DNA damage. These mechanisms also contribute to the regulation of HSC function and are essential to ensure viability after DNA damage. How HSC maintain their quiescent stage during the entire life is still matter of ongoing research. Here we will focus on the molecular mechanisms that regulate HSC function.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

